Patient-reported outcomes in randomised clinical trials of bladder cancer: an updated systematic review
- PMID: 31521149
- PMCID: PMC6744649
- DOI: 10.1186/s12894-019-0518-9
Patient-reported outcomes in randomised clinical trials of bladder cancer: an updated systematic review
Abstract
Background: Despite international recommendations of including patient-reported outcomes (PROs) in randomised clinical trials (RCTs), a 2014 review concluded that few RCTs of bladder cancer (BC) report PRO as an outcome. We therefore aimed to update the 2014 review to synthesise current evidence-based knowledge of PROs from RCTs in BC. A secondary objective was to examine whether quality of PRO reporting has improved over time and to provide evidence-based recommendations for future studies in this area.
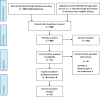
Methods: We conducted a systematic literature search using PubMed/Medline, from April 2014 until June 2018. We included the RCTs identified in the previous review as well as newly published RCTs. Studies were evaluated using a predefined electronic-data extraction form that included information on basic trial demographics, clinical and PRO characteristics and standards of PRO reporting based on recommendation from the International Society of Quality of Life Research.
Results: Since April 2014 only eight new RCTs for BC included PROs as a secondary outcome. In terms of methodology, only the proportion of RCTs documenting the extent of missing PRO data (75% vs 11.1%, p = 0.03) and the identification of PROs in trial protocols (50% vs 0%, p = 0.015) improved. Statistical approaches for dealing with missing data were not reported in most new studies (75%).
Conclusion: Little improvement into the uptake and assessment of PRO as an outcome in RCTs for BC has been made during recent years. Given the increase in (immunotherapy) drug trials with a potential for severe adverse events, there is urgent need to adopt the recommendations and standards available for PRO use in bladder cancer RCTs.
Keywords: Bladder cancer; Outcome measurement; PROs; Quality of life; RCT.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Efficace F, Fayers P, Pusic A, Cemal Y, Yanagawa J, Jacobs M, et al. Quality of patient-reported outcome reporting across cancer randomized controlled trials according to the CONSORT patient-reported outcome extension: a pooled analysis of 557 trials. Cancer. 2015;121(18):3335–3342. doi: 10.1002/cncr.29489. - DOI - PMC - PubMed
-
- U.S. Food and Drug Administration . Guidance for Industry and FDA Staff Qualification Process for Drug Development Tools. 2014.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous