Determination of reliable lung function parameters in intubated mice
- PMID: 31521163
- PMCID: PMC6744631
- DOI: 10.1186/s12931-019-1177-9
Determination of reliable lung function parameters in intubated mice
Abstract
Background: Animal models and, in particular, mice models, are important tools to investigate the pathogenesis of respiratory diseases and to test potential new therapeutic drugs. Lung function measurement is a key step in such investigation. In mice, it is usually performed using forced oscillation technique (FOT), negative pressure-driven forced expiratory (NPFE) and pressure-volume (PV) curve maneuvers. However, these techniques require a tracheostomy, which therefore only allows end-point measurements. Orotracheal intubation has been reported to be feasible and to give reproducible lung function measurements, but the agreement between intubation and tracheostomy generated-data remains to be tested.
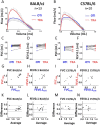
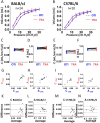
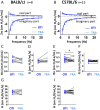
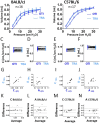
Methods: Using the Flexivent system, we measured lung function parameters (in particular, forced vital capacity (FVC), forced expiratory volume in the first 0.1 s (FEV0.1), compliance (Crs) of the respiratory system, compliance (C) measured using PV loop and an estimate of inspiratory capacity (A)) in healthy intubated BALB/cJ mice and C57BL/6 J mice and compared the results with similar measurements performed in the same mice subsequently tracheostomized after intubation, by means of paired comparison method, correlation and Bland-Altman analysis. The feasibility of repetitive lung function measurements by intubation was also tested.
Results: We identified parameters that are accurately evaluated in intubated animals (i.e., FVC, FEV0.1, Crs, C and A in BALB/cJ and FVC, FEV0.1, and A in C57BL/6 J). Repetitive lung function measurements were obtained in C57BL/6 J mice.
Conclusion: This subset of lung function parameters in orotracheally intubated mice is reliable, thereby allowing relevant longitudinal studies.
Keywords: Asthma; COPD; Lung function; Mice model; Orotracheal intubation.
Conflict of interest statement
Dr. Berger reports grants from Nycomed, grants from Takeda, grants from Fondation du Souffle–Fonds de dotation Recherche en Santé Respiratoire, during the conduct of the study; grants and personal fees from Novartis, personal fees and non-financial support from Chiesi, grants, personal fees and non-financial support from Boehringer Ingelheim, personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from Sanofi, personal fees from Menarinni, personal fees from TEVA, outside the submitted work. In addition, Dr. Berger and Dr. Dupin have a patent (EP N°15152886.6 i.e. New compositions and methods of treating and/or preventing Chronic Obstructive Pulmonary Disease) pending.
Figures





Similar articles
-
Repeated invasive lung function measurements in intubated mice: an approach for longitudinal lung research.Lab Anim. 2011 Apr;45(2):81-9. doi: 10.1258/la.2010.010111. Epub 2011 Feb 28. Lab Anim. 2011. PMID: 21357700
-
Combined forced oscillation and forced expiration measurements in mice for the assessment of airway hyperresponsiveness.Respir Res. 2010 Jun 21;11(1):82. doi: 10.1186/1465-9921-11-82. Respir Res. 2010. PMID: 20565957 Free PMC article.
-
Forced expiration measurements in mouse models of obstructive and restrictive lung diseases.Respir Res. 2017 Jun 19;18(1):123. doi: 10.1186/s12931-017-0610-1. Respir Res. 2017. PMID: 28629359 Free PMC article.
-
[Physiopathology of acute respiratory failure in COPD and asthma].Minerva Anestesiol. 2001 Apr;67(4):198-205. Minerva Anestesiol. 2001. PMID: 11376510 Review. Italian.
-
Invasive and noninvasive lung function measurements in rodents.J Pharmacol Toxicol Methods. 2007 Jan-Feb;55(1):16-26. doi: 10.1016/j.vascn.2006.04.006. Epub 2006 May 9. J Pharmacol Toxicol Methods. 2007. PMID: 16793289 Review.
Cited by
-
Haploinsufficiency of Col5a1 causes intrinsic lung and respiratory changes in a mouse model of classical Ehlers-Danlos syndrome.Physiol Rep. 2022 Apr;10(8):e15275. doi: 10.14814/phy2.15275. Physiol Rep. 2022. PMID: 35439366 Free PMC article.
-
Asthma: The Use of Animal Models and Their Translational Utility.Cells. 2023 Apr 5;12(7):1091. doi: 10.3390/cells12071091. Cells. 2023. PMID: 37048164 Free PMC article. Review.
-
Dual inhibition of the MEK/ERK and PI3K/AKT pathways prevents pulmonary GVHD suppressing perivenulitis and bronchiolitis.Blood Adv. 2023 Jan 10;7(1):106-121. doi: 10.1182/bloodadvances.2021006678. Blood Adv. 2023. PMID: 35468620 Free PMC article.
-
A leak-free head-out plethysmography system to accurately assess lung function in mice.J Appl Physiol (1985). 2022 Jul 1;133(1):104-118. doi: 10.1152/japplphysiol.00835.2021. Epub 2022 May 19. J Appl Physiol (1985). 2022. PMID: 35608203 Free PMC article.
-
N-cadherin antagonism is bronchoprotective in severe asthma models.Sci Adv. 2024 Nov 29;10(48):eadp8872. doi: 10.1126/sciadv.adp8872. Epub 2024 Nov 29. Sci Adv. 2024. PMID: 39612338 Free PMC article.
References
-
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2019. Available from: https://goldcopd.org
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

