Immunohistochemical phenotyping of T cells, granulocytes, and phagocytes in the muscle of cancer patients: association with radiologically defined muscle mass and gene expression
- PMID: 31521204
- PMCID: PMC6744687
- DOI: 10.1186/s13395-019-0209-y
Immunohistochemical phenotyping of T cells, granulocytes, and phagocytes in the muscle of cancer patients: association with radiologically defined muscle mass and gene expression
Abstract
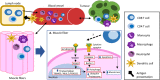
Background: Inflammation is a recognized contributor to muscle wasting. Research in injury and myopathy suggests that interactions between the skeletal muscle and immune cells confer a pro-inflammatory environment that influences muscle loss through several mechanisms; however, this has not been explored in the cancer setting. This study investigated the local immune environment of the muscle by identifying the phenotype of immune cell populations in the muscle and their relationship to muscle mass in cancer patients.
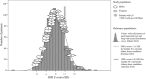
Methods: Intraoperative muscle biopsies were collected from cancer patients (n = 30, 91% gastrointestinal malignancies). Muscle mass was assessed histologically (muscle fiber cross-sectional area, CSA; μm2) and radiologically (lumbar skeletal muscle index, SMI; cm2/m2 by computed tomography, CT). T cells (CD4 and CD8) and granulocytes/phagocytes (CD11b, CD14, and CD15) were assessed by immunohistochemistry. Microarray analysis was conducted in the muscle of a second cancer patient cohort.
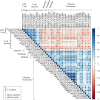
Results: T cells (CD3+), granulocytes/phagocytes (CD11b+), and CD3-CD4+ cells were identified. Muscle fiber CSA (μm2) was positively correlated (Spearman's r = > 0.45; p = < 0.05) with the total number of T cells, CD4, and CD8 T cells and granulocytes/phagocytes. In addition, patients with the smallest SMI exhibited fewer CD8 T cells within their muscle. Consistent with this, further exploration with gene correlation analyses suggests that the presence of CD8 T cells is negatively associated (Pearson's r = ≥ 0.5; p = <0.0001) with key genes within muscle catabolic pathways for signaling (ACVR2B), ubiquitin proteasome (FOXO4, TRIM63, FBXO32, MUL1, UBC, UBB, UBE2L3), and apoptosis/autophagy (CASP8, BECN1, ATG13, SIVA1).
Conclusion: The skeletal muscle immune environment of cancer patients is comprised of immune cell populations from the adaptive and innate immunity. Correlations of T cells, granulocyte/phagocytes, and CD3-CD4+ cells with muscle mass measurements indicate a positive relationship between immune cell numbers and muscle mass status in cancer patients. Further exploration with gene correlation analyses suggests that the presence of CD8 T cells is negatively correlated with components of muscle catabolism.
Keywords: Adaptive immunity; CD8 T cells; Cancer; Computed tomography; Granulocytes; Innate immunity; Muscle biopsy; Muscle catabolism; Muscle mass; Phagocytes; T cells.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Prim. 2018;4:17105. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

