Oral cholera vaccination coverage after the first global stockpile deployment in Haiti, 2014
- PMID: 31521413
- PMCID: PMC10334223
- DOI: 10.1016/j.vaccine.2019.09.018
Oral cholera vaccination coverage after the first global stockpile deployment in Haiti, 2014
Abstract
Introduction: In 2014, an oral cholera vaccine (OCV) campaign targeting 185,314 persons aged ≥1 years was conducted in 3 departments via fixed post and door-to-door strategies. This was the first use of the global OCV stockpile in Haiti.
Methods: We conducted a multi-stage cluster survey to assess departmental OCV coverage. Target population estimates were projected from the 2003 Haiti population census with adjustments for population growth and estimated proportion of pregnant women. In the three departments, we sampled 30/106 enumeration areas (EAs) in Artibonite, 30/244 EAs in Centre, and 20/29 EAs in Ouest; 20 households were systematically sampled in each EA. Household and individual interviews using a standard questionnaire were conducted in each selected household; data on OCV receipt were obtained from vaccination card or verbal report. We calculated OCV campaign coverage estimates and 95% confidence intervals (CIs) accounting for survey design.
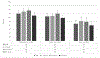
Results: Overall two-dose OCV coverage was 70% (95% CI: 60, 79), 63% (95% CI: 55, 71), and 44% (95% CI: 35, 53) in Artibonite, Centre, and Ouest, respectively. Two-dose coverage was higher in the 1-4 years age group than among those ≥ 15 years in Artibonite (difference: 11%; 95% CI: 5%, 17%) and Ouest (difference: 12%; 95% CI: 3, 20). A higher percentage of children aged 5-14 years received both recommended doses than did those ≥ 15 years (Artibonite: 14% (95% CI: 8%, 19%) difference; Centre: 11% difference (95% CI: 5%, 17%); Ouest: 10% difference (95% CI: 2%, 17%). The most common reason for not receiving any OCV dose was being absent during the campaign or not having heard about vaccination activities.
Conclusions: While coverage estimates in Artibonite and Centre were comparable with other OCV campaigns in Haiti and elsewhere, inadequate social mobilization and outdated population estimates might have contributed to lower coverage in Ouest.
Keywords: Cholera; Cholera vaccine; Diarrhea.
Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Rapport du Reseau National de Surveillance Sites Cholera. http://mspp.gouv.ht/site/downloads/Profil%20statistique%20Cholera%2048em...: Direction d’Epidemiologie de Laboratoire et de Recherches; 2017.
-
- Population MSedl. National Plan for the Elimination of Cholera in Haiti 2013–2022. https://www.paho.org/hq/index.php?option=com_docman&task=doc_download&gi...: National Directorate for Water Supply and Santitation; 2012.
-
- Cholera vaccines: WHO position paper - August 2017. Wkly Epidemiol Rec. 2017; 92: 477–98. - PubMed
-
- Guidance note on the use of Oral Cholera Vaccines for UNICEF. http://www.unicef.org/immunization/files/UNICEF_OCV_Guidance_20_July2012...: UNICEF; 2012.
-
- Bhattacharya SK, Sur D, Ali M, Kanungo S, You YA, Manna B, et al. 5 year efficacy of a bivalent killed whole-cell oral cholera vaccine in Kolkata, India: a cluster-randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 2013;13:1050–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

