Hippocampal-Prefrontal Theta Transmission Regulates Avoidance Behavior
- PMID: 31521441
- PMCID: PMC6842114
- DOI: 10.1016/j.neuron.2019.08.006
Hippocampal-Prefrontal Theta Transmission Regulates Avoidance Behavior
Abstract
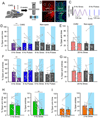
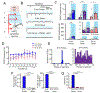
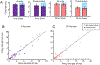
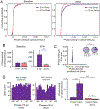
Long-range synchronization of neural oscillations correlates with distinct behaviors, yet its causal role remains unproven. In mice, tests of avoidance behavior evoke increases in theta-frequency (∼8 Hz) oscillatory synchrony between the ventral hippocampus (vHPC) and medial prefrontal cortex (mPFC). To test the causal role of this synchrony, we dynamically modulated vHPC-mPFC terminal activity using optogenetic stimulation. Oscillatory stimulation at 8 Hz maximally increased avoidance behavior compared to 2, 4, and 20 Hz. Moreover, avoidance behavior was selectively increased when 8-Hz stimulation was delivered in an oscillatory, but not pulsatile, manner. Furthermore, 8-Hz oscillatory stimulation enhanced vHPC-mPFC neurotransmission and entrained neural activity in the vHPC-mPFC network, resulting in increased synchrony between vHPC theta activity and mPFC spiking. These data suggest a privileged role for vHPC-mPFC theta-frequency communication in generating avoidance behavior and provide direct evidence that synchronized oscillations play a role in facilitating neural transmission and behavior.
Keywords: anxiety; avoidance; hippocampus; medial prefrontal cortex; optogenetics; oscillations; synchrony; theta.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of Interests:
The authors declare no competing interests.
Figures

References
-
- Ciocchi S, Passecker J, Malagon-Vina H, Mikus N, and Klausberger T (2015). Brain computation. Selective information routing by ventral hippocampal CA1 projection neurons. Science 348, 560–563. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

