Tryptoline-based benzothiazoles re-sensitize MRSA to β-lactam antibiotics
- PMID: 31521461
- PMCID: PMC6779328
- DOI: 10.1016/j.bmc.2019.115095
Tryptoline-based benzothiazoles re-sensitize MRSA to β-lactam antibiotics
Abstract
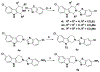
Resistance-modifying agents (RMAs) offer a promising solution to combat bacterial antibiotic resistance. Here we report the discovery and structure-activity relationships of a new class of RMAs with a novel tryptoline-based benzothiazole scaffold. Our most potent compound in this series (4ad) re-sensitizes multiple MRSA strains to cephalosporins at low concentrations (2 μg/mL) and has low mammalian cytotoxicity with a half growth inhibitory concentration (GI50) > 100 μg/mL in human cervical carcinoma (HeLa) cells. In addition, the same core scaffold with different substitutions also gives good antibacterial activity against MRSA.
Keywords: Bacterial antibiotic resistance; Methicillin-resistant Staphylococcus aureus (MRSA); Resistance-modifying agents (RMAs); Structure-activity relationship (SAR); Tryptoline-based benzothiazoles; β-lactam antibiotics.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict and interest
The authors confirm that this article content has no conflict of interest.
Figures
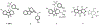





Similar articles
-
Tetracyclic indolines as a novel class of β-lactam-selective resistance-modifying agent for MRSA.Eur J Med Chem. 2017 Jan 5;125:130-142. doi: 10.1016/j.ejmech.2016.09.034. Epub 2016 Sep 10. Eur J Med Chem. 2017. PMID: 27657810 Free PMC article.
-
Synthesis and Bio-Evaluation of Quaternized Fused-β-Carbolines as Anti-MRSA Agents.ChemMedChem. 2025 May 5;20(9):e202400955. doi: 10.1002/cmdc.202400955. Epub 2025 Feb 25. ChemMedChem. 2025. PMID: 39948749
-
Synergistic effect of non-steroidal anti-inflammatory drugs (NSAIDs) on antibacterial activity of cefuroxime and chloramphenicol against methicillin-resistant Staphylococcus aureus.J Glob Antimicrob Resist. 2017 Sep;10:70-74. doi: 10.1016/j.jgar.2017.03.012. Epub 2017 Jun 30. J Glob Antimicrob Resist. 2017. PMID: 28673701
-
Pyrazole-based analogs as potential antibacterial agents against methicillin-resistance staphylococcus aureus (MRSA) and its SAR elucidation.Eur J Med Chem. 2021 Feb 15;212:113134. doi: 10.1016/j.ejmech.2020.113134. Epub 2020 Dec 27. Eur J Med Chem. 2021. PMID: 33395624 Review.
-
Indole-based derivatives as potential antibacterial activity against methicillin-resistance Staphylococcus aureus (MRSA).Eur J Med Chem. 2020 May 15;194:112245. doi: 10.1016/j.ejmech.2020.112245. Epub 2020 Mar 20. Eur J Med Chem. 2020. PMID: 32220687 Review.
Cited by
-
Serendipitous Discovery of a Highly Active and Selective Resistance-Modifying Agent for Colistin-Resistant Gram-Negative Bacteria.ACS Omega. 2022 Mar 30;7(14):12442-12446. doi: 10.1021/acsomega.2c01530. eCollection 2022 Apr 12. ACS Omega. 2022. PMID: 35449921 Free PMC article.
-
Structure-activity relationship studies of [1,2,5]oxadiazolo[3,4-b]pyrazine-containing polymyxin-selective resistance-modifying agents.Bioorg Med Chem Lett. 2022 Sep 15;72:128878. doi: 10.1016/j.bmcl.2022.128878. Epub 2022 Jul 3. Bioorg Med Chem Lett. 2022. PMID: 35788034 Free PMC article.
-
Review on the Developments of Benzothiazole-containing Antimicrobial Agents.Curr Top Med Chem. 2022;22(32):2630-2659. doi: 10.2174/1568026623666221207161752. Curr Top Med Chem. 2022. PMID: 36503470 Review.
References
-
- Davies J, Microbiologia 1996, 12, 9–16 - PubMed
-
- Bush K, Clin Microbiol Infect 2004, 10 Suppl 4, 10–17. - PubMed
-
- Stryjewski ME; Corey GR, Clin Infect Dis 2014, 58 Suppl 1, S10–9. - PubMed
-
- D’Costa VM; King CE; Kalan L; Morar M; Sung WW; Schwarz C; Froese D; Zazula G; Calmels F; Debruyne R; Golding GB; Poinar HN; Wright GD, Nature 2011, 477, 457–461. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

