CRISPR-based rapid and ultra-sensitive diagnostic test for Mycobacterium tuberculosis
- PMID: 31522608
- PMCID: PMC6758691
- DOI: 10.1080/22221751.2019.1664939
CRISPR-based rapid and ultra-sensitive diagnostic test for Mycobacterium tuberculosis
Abstract
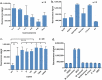
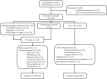
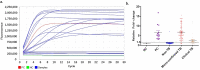
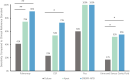
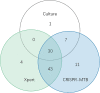
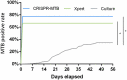
Rapid and simple-to-use diagnostic methods for tuberculosis are urgently needed. Recent development has unveiled the diagnostic power of the CRISPR system in the detection of viral infections. However, its potential use in detecting the Mycobacterium tuberculosis complex (MTB) remained unexplored. We developed a rapid CRISPR-based assay for TB detection and conducted a retrospective cohort study of 179 patients to evaluate the CRISPR-MTB test for identifying MTB in various forms of direct clinical samples. Its diagnostic performance was compared, in parallel with culture and the GeneXpert MTB/RIF assay (Xpert). The CRISPR-MTB test is highly sensitive with a near single-copy sensitivity, demands less sample input and offers shorter turnaround time than Xpert. When evaluated in the clinical cohort of both pulmonary and extra-pulmonary tuberculosis, the CRISPR-MTB test exhibited an overall improved sensitivity over both culture (79% vs 33%) and Xpert (79% vs 66%), without comprise in specificity (62/63, 98%). The CRISPR-MTB test exhibits an improved overall diagnostic performance over culture and Xpert across a variety of sample types, and offers great potential as a new diagnostic technique for both pulmonary and extra-pulmonary tuberculosis.
Keywords: complex (MTB); CRISPR-MTB; GeneXpert MTB/RIF; diagnosis; tuberculosis.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures

References
-
- Organization WH . Global Tuberculosis Report 2017. World Health Organization; 2018.
-
- WHO . Policy statement: automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system. Department/Division; 2011. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
