UK vaccines network: Mapping priority pathogens of epidemic potential and vaccine pipeline developments
- PMID: 31522809
- PMCID: PMC7127063
- DOI: 10.1016/j.vaccine.2019.09.009
UK vaccines network: Mapping priority pathogens of epidemic potential and vaccine pipeline developments
Abstract
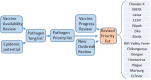
During the 2013-2016 Ebola outbreak in West Africa an expert panel was established on the instructions of the UK Prime Minister to identify priority pathogens for outbreak diseases that had the potential to cause future epidemics. A total of 13 priority pathogens were identified, which led to the prioritisation of spending in emerging diseases vaccine research and development from the UK. This meeting report summarises the process used to develop the UK pathogen priority list, compares it to lists generated by other organisations (World Health Organisation, National Institutes of Allergy and Infectious Diseases) and summarises clinical progress towards the development of vaccines against priority diseases. There is clear technical progress towards the development of vaccines. However, the availability of these vaccines will be dependent on sustained funding for clinical trials and the preparation of clinically acceptable manufactured material during inter-epidemic periods.
Keywords: Epidemic; Outbreak; Pathogen; Priority; UKVN; Vaccine.
Copyright © 2019.
Figures

References
-
- Henao-Restrepo A.M., Camacho A., Longini I.M., Watson C.H., Edmunds W.J., Egger M. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!) The Lancet. 2017;389:505–518. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

