UVB-Induced Tumor Heterogeneity Diminishes Immune Response in Melanoma
- PMID: 31522890
- PMCID: PMC6863386
- DOI: 10.1016/j.cell.2019.08.032
UVB-Induced Tumor Heterogeneity Diminishes Immune Response in Melanoma
Abstract
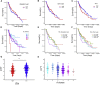
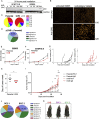
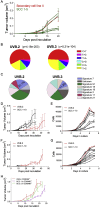
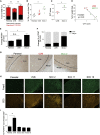
Although clonal neo-antigen burden is associated with improved response to immune therapy, the functional basis for this remains unclear. Here we study this question in a novel controlled mouse melanoma model that enables us to explore the effects of intra-tumor heterogeneity (ITH) on tumor aggressiveness and immunity independent of tumor mutational burden. Induction of UVB-derived mutations yields highly aggressive tumors with decreased anti-tumor activity. However, single-cell-derived tumors with reduced ITH are swiftly rejected. Their rejection is accompanied by increased T cell reactivity and a less suppressive microenvironment. Using phylogenetic analyses and mixing experiments of single-cell clones, we dissect two characteristics of ITH: the number of clones forming the tumor and their clonal diversity. Our analysis of melanoma patient tumor data recapitulates our results in terms of overall survival and response to immune checkpoint therapy. These findings highlight the importance of clonal mutations in robust immune surveillance and the need to quantify patient ITH to determine the response to checkpoint blockade.
Keywords: anti-tumor immunity; cancer neoantigens; checkpoint immunotherapy; intra-tumor heterogeneity; melanoma; mouse model; mutational load.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
C.S. declares the following receipt of grants/research support: Pfizer, AstraZeneca, BMS, Roche Ventana. Receipt of honoraria, consultancy, or SAB Member fees: Pfizer, Novartis, GlaxoSmithKline, MSD, BMS, Celgene, AstraZeneca, Illumina, Sarah Canon Research Institute, Genentech, Roche-Ventana, GRAIL, Medicxi Advisor for Dynamo Therapeutics. Stock shareholder: Apogen Biotechnologies, Epic Bioscience, GRAIL. Co-Founder & stock options: Achilles Therapeutics. K.L. reports speaker fees from Roche Tissue Diagnostics and patents pending on indel burden as a predictor of checkpoint inhibitor response and targeting of frameshift neoantigens for personalised immunotherapy.
Figures










Comment in
-
Paradoxical roles of mutational load as a determinant of anticancer immunity.Nat Rev Clin Oncol. 2019 Nov;16(11):659. doi: 10.1038/s41571-019-0282-5. Nat Rev Clin Oncol. 2019. PMID: 31562491 No abstract available.
-
Tumour heterogeneity determines immune response.Nat Rev Immunol. 2019 Nov;19(11):662-663. doi: 10.1038/s41577-019-0230-8. Nat Rev Immunol. 2019. PMID: 31586160 No abstract available.
-
Tumor Neoantigens: When Too Much of a Good Thing Is Bad.Cancer Cell. 2019 Nov 11;36(5):466-467. doi: 10.1016/j.ccell.2019.10.009. Cancer Cell. 2019. PMID: 31715129
References
-
- Alexandrov L.B., Nik-Zainal S., Wedge D.C., Aparicio S.A., Behjati S., Biankin A.V., Bignell G.R., Bolli N., Borg A., Børresen-Dale A.L., Australian Pancreatic Cancer Genome Initiative. ICGC Breast Cancer Consortium. ICGC MMML-Seq Consortium. ICGC PedBrain Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. - PMC - PubMed
-
- Alter G., Malenfant J.M., Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods. 2004;294:15–22. - PubMed
-
- Arce Vargas F., Furness A.J.S., Solomon I., Joshi K., Mekkaoui L., Lesko M.H., Miranda Rota E., Dahan R., Georgiou A., Sledzinska A., Melanoma TRACERx Consortium. Renal TRACERx Consortium. Lung TRACERx Consortium Fc-Optimized Anti-CD25 Depletes Tumor-Infiltrating Regulatory T Cells and Synergizes with PD-1 Blockade to Eradicate Established Tumors. Immunity. 2017;46:577–586. - PMC - PubMed
-
- Bar-On L., Birnberg T., Kim K.W., Jung S. Dendritic cell-restricted CD80/86 deficiency results in peripheral regulatory T-cell reduction but is not associated with lymphocyte hyperactivation. Eur. J. Immunol. 2011;41:291–298. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

