A Complex of U1 snRNP with Cleavage and Polyadenylation Factors Controls Telescripting, Regulating mRNA Transcription in Human Cells
- PMID: 31522989
- PMCID: PMC6874754
- DOI: 10.1016/j.molcel.2019.08.007
A Complex of U1 snRNP with Cleavage and Polyadenylation Factors Controls Telescripting, Regulating mRNA Transcription in Human Cells
Abstract
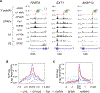
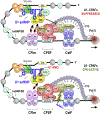
Full-length transcription in the majority of human genes depends on U1 snRNP (U1) to co-transcriptionally suppress transcription-terminating premature 3' end cleavage and polyadenylation (PCPA) from cryptic polyadenylation signals (PASs) in introns. However, the mechanism of this U1 activity, termed telescripting, is unknown. Here, we captured a complex, comprising U1 and CPA factors (U1-CPAFs), that binds intronic PASs and suppresses PCPA. U1-CPAFs are distinct from U1-spliceosomal complexes; they include CPA's three main subunits, CFIm, CPSF, and CstF; lack essential splicing factors; and associate with transcription elongation and mRNA export complexes. Telescripting requires U1:pre-mRNA base-pairing, which can be disrupted by U1 antisense oligonucleotide (U1 AMO), triggering PCPA. U1 AMO remodels U1-CPAFs, revealing changes, including recruitment of CPA-stimulating factors, that explain U1-CPAFs' switch from repressive to activated states. Our findings outline this U1 telescripting mechanism and demonstrate U1's unique role as central regulator of pre-mRNA processing and transcription.
Keywords: -end processing; U1 snRNP; cleavage and polyadenylation; mRNA 3ʹ; telescripting; transcription elongation; transcription termination.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures



References
-
- Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait BT, Sali A, and Rout MP (2007). The molecular architecture of the nuclear pore complex. Nature 450, 695–701. - PubMed
-
- Andersen PR, Domanski M, Kristiansen MS, Storvall H, Ntini E, Verheggen C, Schein A, Bunkenborg J, Poser I, Hallais M, Sandberg R, Hyman A, LaCava J, Rout MP, Andersen JS, Bertrand E, and Jensen TH (2013). The human cap-binding complex is functionally connected to the nuclear RNA exosome. Nat Struct Mol Biol 20, 1367–1376. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

