Novel insights into the treatment of complement-mediated hemolytic anemias
- PMID: 31523413
- PMCID: PMC6734604
- DOI: 10.1177/2040620719873321
Novel insights into the treatment of complement-mediated hemolytic anemias
Abstract
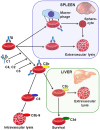
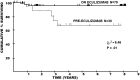
Complement-mediated hemolytic anemias can either be caused by deficiencies in regulatory complement components or by autoimmune pathogenesis that triggers inappropriate complement activation. In paroxysmal nocturnal hemoglobinuria (PNH) hemolysis is entirely complement-driven. Hemolysis is also thought to be complement-dependent in cold agglutinin disease (CAD) and in paroxysmal cold hemoglobinuria (PCH), whereas warm antibody autoimmune hemolytic anemia (wAIHA) is a partially complement-mediated disorder, depending on the subtype of wAIHA and the extent of complement activation. The pathophysiology, clinical presentation, and current therapies for these diseases are reviewed in this article. Novel, complement-directed therapies are being rapidly developed. Therapeutic terminal complement inhibition using eculizumab has revolutionized the therapy and prognosis in PNH but has proved less efficacious in CAD. Upstream complement modulation is currently being investigated and appears to be a highly promising therapy, and two such agents have entered phase II and III trials. Of these, the anti-C1s monoclonal antibody sutimlimab has shown favorable activity in CAD, while the anti-C3 cyclic peptide pegcetacoplan appears to be promising in PNH as well as CAD, and may also have a therapeutic potential in wAIHA.
Keywords: autoimmune hemolytic anemia; cold agglutinin disease; complement; complement inhibitors; paroxysmal nocturnal hemoglobinuria; therapy.
Conflict of interest statement
Conflict of interest statement: The authors did not receive any funding for this work. Outside this work, S. Berentsen has received research funding from Mundipharma, consulting fees from Apellis, Bioverativ, Momenta Pharmaceuticals and True North Therapeutics, and travel grants and lecture honoraria from Alexion, Apellis, Bioverativ and Janssen-Cilag. A. Hill has received honoraria from Alexion, Apellis, Bioverativ, and Novartis. Q.A. Hill has received research support from Alexion and honoraria for lecturing or advisory work from Novartis, Shire, and Bioverativ. T.H.A. Tvedt has received advisory board honoraria from Ablynx and Alexion and lecture honoraria from Alexion and Novartis. M. Michel has advisory board honoraria from Alexion, Apellis, and Rigel.
Figures


References
-
- Risitano AM. Therapeutic complement modulation for hematological diseases: where we stand and where we are going. Semin Hematol 2018; 55: 113–117. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

