Comparative transcriptomics reveals the difference in early endosperm development between maize with different amylose contents
- PMID: 31523504
- PMCID: PMC6717500
- DOI: 10.7717/peerj.7528
Comparative transcriptomics reveals the difference in early endosperm development between maize with different amylose contents
Abstract
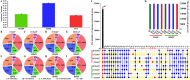
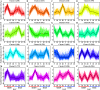
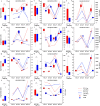
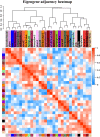
In seeds, the endosperm is a crucial organ that plays vital roles in supporting embryo development and determining seed weight and quality. Starch is the predominant storage carbohydrate of the endosperm and accounts for ∼70% of the mature maize kernel weight. Nonetheless, because starch biosynthesis is a complex process that is orchestrated by multiple enzymes, the gene regulatory networks of starch biosynthesis, particularly amylose and amylopectin biosynthesis, have not been fully elucidated. Here, through high-throughput RNA sequencing, we developed a temporal transcriptome atlas of the endosperms of high-amylose maize and common maize at 5-, 10-, 15- and 20-day after pollination and found that 21,986 genes are involved in the programming of the high-amylose and common maize endosperm. A coexpression analysis identified multiple sequentially expressed gene sets that are closely correlated with cellular and metabolic programmes and provided valuable insight into the dynamic reprogramming of the transcriptome in common and high-amylose maize. In addition, a number of genes and transcription factors were found to be strongly linked to starch synthesis, which might help elucidate the key mechanisms and regulatory networks underlying amylose and amylopectin biosynthesis. This study will aid the understanding of the spatiotemporal patterns and genetic regulation of endosperm development in different types of maize and provide valuable genetic information for the breeding of starch varieties with different contents.
Keywords: Endosperm; Gene expression; Maize; RNA-sequence; Starch metabolism.
Conflict of interest statement
The authors declare there are no competing interests.
Figures



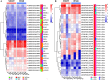
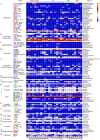
References
-
- Ai Y, Jane J-L. Chapter 3—understanding starch structure and functionality. In: Sjöö M, Nilsson L, editors. Starch in food. Second Edition. Sawston & Cambridge: Woodhead Publishing; 2018. pp. 151–178. - DOI
-
- Bagga S, Adams HP, Rodriguez FD, Kemp JD, Sengupta-Gopalan C. Coexpression of the maize delta-zein and beta-zein genes results in stable accumulation of delta-zein in endoplasmic reticulum-derived protein bodies formed by beta-zein. The Plant Cell. 1997;9:1683–1696. doi: 10.1105/tpc.9.9.1683. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

