Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels
- PMID: 31523707
- PMCID: PMC6731072
- DOI: 10.1126/sciadv.aaw2459
Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels
Abstract
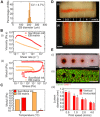
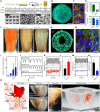
Engineering organ-specific tissues for therapeutic applications is a grand challenge, requiring the fabrication and maintenance of densely cellular constructs composed of ~108 cells/ml. Organ building blocks (OBBs) composed of patient-specific-induced pluripotent stem cell-derived organoids offer a pathway to achieving tissues with the requisite cellular density, microarchitecture, and function. However, to date, scant attention has been devoted to their assembly into 3D tissue constructs. Here, we report a biomanufacturing method for assembling hundreds of thousands of these OBBs into living matrices with high cellular density into which perfusable vascular channels are introduced via embedded three-dimensional bioprinting. The OBB matrices exhibit the desired self-healing, viscoplastic behavior required for sacrificial writing into functional tissue (SWIFT). As an exemplar, we created a perfusable cardiac tissue that fuses and beats synchronously over a 7-day period. Our SWIFT biomanufacturing method enables the rapid assembly of perfusable patient- and organ-specific tissues at therapeutic scales.
Figures


References
-
- Takebe T., Sekine K., Enomura M., Koike H., Kimura M., Ogaeri T., Zhang R.-R., Ueno Y., Zheng Y.-W., Koike N., Aoyama S., Adachi Y., Taniguchi H., Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499, 481–484 (2013). - PubMed
-
- Zimmermann W.-H., Melnychenko I., Wasmeier G., Didié M., Naito H., Nixdorff U., Hess A., Budinsky L., Brune K., Michaelis B., Dhein S., Schwoerer A., Ehmke H., Eschenhagen T., Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat. Med. 12, 452–458 (2006). - PubMed
-
- Carrier R. L., Papadaki M., Rupnick M., Schoen F. J., Bursac N., Langer R., Freed L. E., Vunjak-Novakovic G., Cardiac tissue engineering: Cell seeding, cultivation parameters, and tissue construct characterization. Biotechnol. Bioeng. 64, 580–589 (1999). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

