Versatile Peptide Macrocyclization with Diels-Alder Cycloadditions
- PMID: 31523967
- PMCID: PMC7745206
- DOI: 10.1021/jacs.9b07578
Versatile Peptide Macrocyclization with Diels-Alder Cycloadditions
Abstract
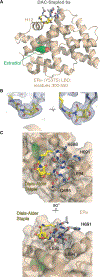
Macrocyclization can improve bioactive peptide ligands through preorganization of molecular topology, leading to improvement of pharmacologic properties like binding affinity, cell permeability, and metabolic stability. Here we demonstrate that Diels-Alder [4 + 2] cycloadditions can be harnessed for peptide macrocyclization and stabilization within a range of peptide scaffolds and chemical environments. Diels-Alder cyclization of diverse diene-dienophile reactive pairs proceeds rapidly, in high yield and with tunable stereochemical preferences on solid-phase or in aqueous solution. This reaction can be applied alone or in concert with other stabilization chemistries, such as ring-closing olefin metathesis, to stabilize loop, turn, and α-helical secondary structural motifs. NMR and molecular dynamics studies of model loop peptides confirmed preferential formation of endo cycloadduct stereochemistry, imparting significant structural rigidity to the peptide backbone that resulted in augmented protease resistance and increased biological activity of a Diels-Alder cyclized (DAC) RGD peptide. Separately, we demonstrated the stabilization of DAC α-helical peptides derived from the ERα-binding protein SRC2. We solved a 2.25 Å cocrystal structure of one DAC helical peptide bound to ERα, which unequivocally corroborated endo stereochemistry of the resulting Diels-Alder adduct, and confirmed that the unique architecture of stabilizing motifs formed with this chemistry can directly contribute to target binding. These data establish Diels-Alder cyclization as a versatile approach to stabilize diverse protein structural motifs under a range of chemical environments.
Conflict of interest statement
Notes
The authors declare no competing financial interests.
Figures

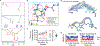
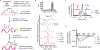

References
-
- Hill TA; Shepherd NE; Diness F; Fairlie DP, Constraining Cyclic Peptides To Mimic Protein Structure Motifs. Angewandte Chemie International Edition 2014, 53 (48), 13020–13041. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

