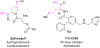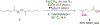Fenton-Inspired C-H Functionalization: Peroxide-Directed C-H Thioetherification
- PMID: 31524395
- PMCID: PMC7423317
- DOI: 10.1021/acs.joc.9b01979
Fenton-Inspired C-H Functionalization: Peroxide-Directed C-H Thioetherification
Abstract
Substoichiometric iron mediates the thioetherification of unactivated aliphatic C-H bonds directed by resident silylperoxides. Upon exposure to a catalytic amount of iron(II) triflate, TIPS-protected peroxides bearing primary, secondary, and tertiary C-H sites undergo chemoselective thioetherification of remote C-H bonds with diaryl disulfides. The reaction demonstrates a broad substrate scope and functional group tolerance without the use of any noble metal additives. Mechanistic experiments suggest that the reaction proceeds through 1,5-H atom abstraction by a hydroxyl radical generated with iron.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Prilezhaeva EN Sulfones and sulfoxides in the total synthesis of biologically active natural compounds. Russ. Chem. Rev 2000, 69, 367.
-
- Trost BM; Conway WP; Strege PE; Dietsche TJ New Synthetic reactions. Alkylative elimination. J. Am. Chem. Soc 1974, 96, 7165.
-
- Evans DA; Andrews GC Allylic sulfoxides. Useful intermediates in organic synthesis. Acc. Chem. Res 1974, 7, 147.
-
- Engstrom KM; Mendoza MR; Navarro-Villalobos M; Gin DY Total Synthesis of (+)-Pyrenolide D. Angew. Chem., Int. Ed 2001, 40, 1128. - PubMed
-
- de Lucchi O; Miotti U; Modena G Org. React 1991, 157.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources