Dietary cranberry suppressed colonic inflammation and alleviated gut microbiota dysbiosis in dextran sodium sulfate-treated mice
- PMID: 31524900
- PMCID: PMC6800821
- DOI: 10.1039/c9fo01537j
Dietary cranberry suppressed colonic inflammation and alleviated gut microbiota dysbiosis in dextran sodium sulfate-treated mice
Abstract
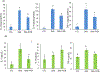
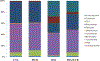
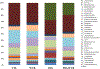
Increased consumption of fruits may decrease the risk of chronic inflammatory diseases including inflammatory bowel disease (IBD). Gut microbiota dysbiosis plays an important etiological role in IBD. However, the mechanisms of action underlying the anti-inflammatory effects of dietary cranberry (Vaccinium macrocarpon) in the colon and its role on gut microbiota were unclear. In this study, we determined the anti-inflammatory efficacy of whole cranberry in a mouse model of dextran sodium sulfate (DSS)-induced colitis, as well as its effects on the structure of gut microbiota. The results showed that dietary cranberry significantly decreased the severity of colitis in DSS-treated mice, evidenced by increased colon length, and decreased disease activity and histologic score of colitis in DSS-treated mice compared to the positive control group (p < 0.05). Moreover, the colonic levels of pro-inflammatory cytokine (IL-1β, IL-6 and TNF-α) were significantly reduced by cranberry supplementation (p < 0.05). Analysis of the relative abundance of fecal microbiota in phylum and genus levels revealed that DSS treatment significantly altered the microbial structure of fecal microbiota in mice. α diversity was significantly decreased in the DSS group, compared to the healthy control group. But, cranberry treatment significantly improved DSS-induced decline in α-diversity. Moreover, cranberry treatment partially reversed the change of gut microbiota in colitic mice by increasing the abundance of potential beneficial bacteria, for example, Lactobacillus and Bifidobacterium, and decreasing the abundance of potential harmful bacteria, such as Sutterella and Bilophila. Overall, our results for the first time demonstrated that modification of gut microbiota by dietary whole cranberry might contribute to its inhibitory effects against the development of colitis in DSS-treated mice.
Figures
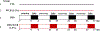




Similar articles
-
Dietary Intake of Whole Strawberry Inhibited Colonic Inflammation in Dextran-Sulfate-Sodium-Treated Mice via Restoring Immune Homeostasis and Alleviating Gut Microbiota Dysbiosis.J Agric Food Chem. 2019 Aug 21;67(33):9168-9177. doi: 10.1021/acs.jafc.8b05581. Epub 2019 Mar 5. J Agric Food Chem. 2019. PMID: 30810035
-
Dihydromyricetin improves DSS-induced colitis in mice via modulation of fecal-bacteria-related bile acid metabolism.Pharmacol Res. 2021 Sep;171:105767. doi: 10.1016/j.phrs.2021.105767. Epub 2021 Jul 14. Pharmacol Res. 2021. PMID: 34273490
-
Flaxseed oligosaccharides alleviate DSS-induced colitis through modulation of gut microbiota and repair of the intestinal barrier in mice.Food Funct. 2020 Sep 23;11(9):8077-8088. doi: 10.1039/d0fo01105c. Food Funct. 2020. PMID: 32856645
-
Impact of Cranberries on Gut Microbiota and Cardiometabolic Health: Proceedings of the Cranberry Health Research Conference 2015.Adv Nutr. 2016 Jul 15;7(4):759S-70S. doi: 10.3945/an.116.012583. Print 2016 Jul. Adv Nutr. 2016. PMID: 27422512 Free PMC article. Review.
-
Effects of Vitamin D Supplementation on Inflammation, Colonic Cell Kinetics, and Microbiota in Colitis: A Review.Molecules. 2020 May 14;25(10):2300. doi: 10.3390/molecules25102300. Molecules. 2020. PMID: 32422882 Free PMC article. Review.
Cited by
-
Structural characteristics of locust bean gum hydrolysate and its alleviating effect on dextran sulfate sodium-induced colitis.Front Microbiol. 2022 Aug 10;13:985725. doi: 10.3389/fmicb.2022.985725. eCollection 2022. Front Microbiol. 2022. PMID: 36033869 Free PMC article.
-
Beneficial Role of Fruits, Their Juices, and Freeze-Dried Powders on Inflammatory Bowel Disease and Related Dysbiosis.Plants (Basel). 2021 Dec 21;11(1):4. doi: 10.3390/plants11010004. Plants (Basel). 2021. PMID: 35009009 Free PMC article. Review.
-
Chemopreventive Effect of Dietary Anthocyanins against Gastrointestinal Cancers: A Review of Recent Advances and Perspectives.Int J Mol Sci. 2020 Sep 8;21(18):6555. doi: 10.3390/ijms21186555. Int J Mol Sci. 2020. PMID: 32911639 Free PMC article. Review.
-
Combating Escherichia coli O157:H7 with Functionalized Chickpea-Derived Antimicrobial Peptides.Adv Sci (Weinh). 2023 Feb;10(6):e2205301. doi: 10.1002/advs.202205301. Epub 2022 Dec 23. Adv Sci (Weinh). 2023. PMID: 36563134 Free PMC article.
-
Cancer-Preventive Role of Bone Marrow-Derived Mesenchymal Stem Cells on Colitis-Associated Colorectal Cancer: Roles of Gut Microbiota Involved.Front Cell Dev Biol. 2021 Jun 4;9:642948. doi: 10.3389/fcell.2021.642948. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34150751 Free PMC article.
References
-
- Ng SC, et al., Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet, 2018. 390(10114): p. 2769–2778. - PubMed
-
- Xavier RJ and Podolsky DK, Unravelling the pathogenesis of inflammatory bowel disease. Nature, 2007. 448(7152): p. 427–34. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

