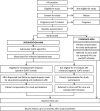
Simplified clinical algorithm for identifying patients eligible for same-day HIV treatment initiation (SLATE): Results from an individually randomized trial in South Africa and Kenya
- PMID: 31525187
- PMCID: PMC6746347
- DOI: 10.1371/journal.pmed.1002912
Simplified clinical algorithm for identifying patients eligible for same-day HIV treatment initiation (SLATE): Results from an individually randomized trial in South Africa and Kenya
Abstract
Background: The World Health Organization recommends "same-day" initiation of antiretroviral therapy (ART) for HIV patients who are eligible and ready. Identifying efficient, safe, and feasible procedures for determining same-day eligibility and readiness is now a priority. The Simplified Algorithm for Treatment Eligibility (SLATE) study evaluated a clinical algorithm that allows healthcare workers to determine eligibility for same-day treatment and to initiate ART at the patient's first clinic visit.
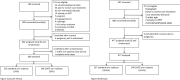
Methods and findings: SLATE was an individually randomized trial at three outpatient clinics in urban settlements in Johannesburg, South Africa and three hospital clinics in western Kenya. Adult, nonpregnant, HIV-positive, ambulatory patients presenting for any HIV care, including HIV testing, but not yet on ART were enrolled and randomized to the SLATE algorithm arm or standard care. The SLATE algorithm used four screening tools-a symptom self-report, medical history questionnaire, physical examination, and readiness assessment-to ascertain eligibility for same-day initiation or refer for further care. Follow-up was by record review, and analysis was conducted by country. We report primary outcomes of 1) ART initiation ≤28 days and 2) initiation ≤28 days and retention in care ≤8 months of enrollment. From March 7, 2017 to April 17, 2018, we enrolled 600 patients (median [IQR] age 34 [29-40] and CD4 count 286 [128-490]; 63% female) in South Africa and 477 patients in Kenya (median [IQR] age 35 [29-43] and CD4 count 283 [117-541]; 58% female). In the intervention arm, 78% of patients initiated ≤28 days in South Africa, compared to 68% in the standard arm (risk difference [RD] [95% confidence interval (CI)] 10% [3%-17%]); in Kenya, 94% of intervention-arm patients initiated ≤28 days compared to 89% in the standard arm (6% [0.5%-11%]). By 8 months in South Africa, 161/298 (54%) intervention-arm patients had initiated and were retained, compared to 146/302 (48%) in the standard arm (6% [(2% to 14%]). By 8 months in Kenya, the corresponding retention outcomes were identical in both arms (137/240 [57%] of intervention-arm patients and 136/237 [57%] of standard-arm patients). Limitations of the trial included limited geographic representativeness, exclusion of patients too ill to participate, missing viral load data, greater study fidelity to the algorithm than might be achieved in standard care, and secular changes in standard care over the course of the study.
Conclusions: In South Africa, the SLATE algorithm increased uptake of ART within 28 days by 10% and showed a numerical increase (6%) in retention at 8 months. In Kenya, the algorithm increased uptake of ART within 28 days by 6% but found no difference in retention at 8 months. Eight-month retention was poor in both arms and both countries. These results suggest that a simple structured algorithm for same-day treatment initiation procedures is feasible and can increase and accelerate ART uptake but that early retention on treatment remains problematic.
Trial registration: Clinicaltrials.gov NCT02891135, registered September 1, 2016. First participant enrolled March 6, 2017 in South Africa and July 13, 2017 in Kenya.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: SR is on the editorial board of PLOS Medicine. BL and ATB are on the editorial board of PLOS ONE. WDFV sits on antiretroviral initiation guideline committees both local and international, has accepted speaking honoraria from multiple manufacturers of antiretrovirals, and is on several of their advisory boards. The remaining authors declare that they have no competing interests.
Figures

References
-
- World Health Organization. Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. Geneva: World Health Organization; 2017. Available from: http://www.who.int/hiv/pub/guidelines/advanced-HIV-disease/en/. - PubMed
-
- National Department of Health. Same-day antiretroviral therapy (ART) initiation for HIV positive patients Pretoria: National Department of Health; 2017.
-
- Ministry of Health and National AIDS and STI Control Program (NASCOP). Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection in Kenya 2018 Edition Nairobi, Kenya: NASCOP; 2018.
-
- Long LC, Maskew M, Brennan AT, Mongwenyana C, Nyoni C, Malete G, et al. Initiating antiretroviral therapy for HIV at a patient’s first clinic visit: a cost-effectiveness analysis of the rapid initiation of treatment randomized controlled trial. AIDS. 2017;31: 1611–19. 10.1097/QAD.0000000000001528 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

