Clinical features of neuroendocrine prostate cancer
- PMID: 31525487
- PMCID: PMC6803064
- DOI: 10.1016/j.ejca.2019.08.011
Clinical features of neuroendocrine prostate cancer
Abstract
Background: Neuroendocrine prostate cancer (NEPC) is an aggressive variant of prostate cancer that may arise de novo or in patients previously treated with hormonal therapies for prostate adenocarcinoma as a mechanism of resistance. Despite being important to recognise, the clinical features of NEPC are poorly defined and could help guide when to perform a biopsy to look for NEPC histologic transformation.
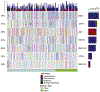
Methods: We reviewed baseline, treatment and outcome data of 87 patients with metastatic prostate cancer and tumour biopsy confirming NEPC histology. Forty-seven (54.0%) NEPC cases presented de novo, and 40 (46.0%) were therapy-related (t-NEPC). Thirty-six (41.4%) were classified as pure small-cell carcinoma, and 51 (58.6%) demonstrated mixed features with both small-cell carcinoma and adenocarcinoma present. Genomic data were available for 47 patients.
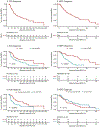
Results: The median age at time of NEPC was 68.1 years, median prostate-specific antigen (PSA) was 1.20 ng/ml (0.14 ng/mL small-cell carcinoma, 1.55 ng/mL mixed carcinoma) and sites of metastases included bone (72.6%), lymph node (47.0%), and viscera (65.5%). Median time from adenocarcinoma to t-NEPC diagnosis was 39.7 months (range, 24.5-93.8) with a median of two lines of prior systemic therapy. Platinum chemotherapy was used to treat 57.5% of patients, with a median progression-free survival of 3.9 months. Small-cell carcinoma was associated with worse overall survival (OS) than mixed histology (8.9 months from NEPC diagnosis versus 26.1 months, P < 0.001). Median OS of de novo NEPC was shorter than that of t-NEPC (16.8 months from prostate cancer diagnosis versus 53.5 months, P = 0.043). An average PSA rise per month of ≤0.7 ng/ml before t-NEPC; elevated lactate dehydrogenase levels, RB1 and TP53 loss and liver metastases were poor prognostic features.
Conclusions: We describe the clinical features of a cohort of patients with NEPC. These characteristics may inform future diagnostic strategies.
Keywords: Aggressive variant; Neuroendocrine prostate cancer; Small-cell carcinoma; Treatment resistance.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement
V.C. received speaker honoraria or travel support from Astellas, Janssen-Cilag, Ipsen Fund and Sanofi Gemzyme and consulting fee from Bayer. S.T. has served as consultant/advisory board member for Medivation, Astellas Pharma, Dendreon, Janssen, Bayer, Genentech, Endocyte, Immunomeidics, Karyiopharm Therapeutics, Abbvie, Tolmar, QED, Amgen, Sanofi, Pfizer, and has received research funding (Inst) from Lilly, Sanofi, Janssen, Astellas Pharma, Progenics, Millenium, Amgen, Brystol-Myers Squibb, Dendreon, Rexahn Pharamceuticals, Bayer, Genentech, Newlink Genetics,Inovio Pharamceuticals, AstraZaneca, Immunomedics, Novartis, AVEO, Boehringer Ingelheim, Merck, Stem CentRx, Karyopharm Therapeutics, Abbvie, Medivation, Endocyte, Exelixis, Clovis Oncology, and has received speaker honoraria or travel support from Sanofi, Immunomedics, and Amgen. H.B. has served as a consultant/advisory board member for Janssen, Sanofi and Astellas and has received research funding from Janssen Oncology (Inst), AbbVie / Stem-centrx, Eli Lilly (Inst) and Millennium Pharmaceuticals (Inst). No potential conflicts of interest were disclosed by the other authors.
Figures


References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics. CA A Cancer J Clin 2018;68:7–30. 2018. - PubMed
-
- Wang W, Epstein JI. Small-cell carcinoma of the prostate. A morphologic and immunohistochemical study of 95 cases. Am J Surg Pathol 2008;32:65–71. - PubMed
-
- Papandreou CN, Daliani DD, Thall PF, et al. Results of a phase II study with doxorubicin, etoposide, and cisplatin in patients with fully characterized small-cell carcinoma of the prostate. J Clin Oncol 2002;20(14):3072–80. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

