Role of the dynamic tumor microenvironment in controversies regarding immune checkpoint inhibitors for the treatment of non-small cell lung cancer (NSCLC) with EGFR mutations
- PMID: 31526368
- PMCID: PMC6745797
- DOI: 10.1186/s12943-019-1062-7
Role of the dynamic tumor microenvironment in controversies regarding immune checkpoint inhibitors for the treatment of non-small cell lung cancer (NSCLC) with EGFR mutations
Abstract
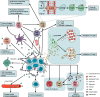
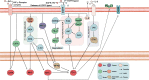
Immunotherapy has been incorporated into the first- and second-line treatment strategies for non-small cell lung cancer (NSCLC), profoundly ushering in a new treatment landscape. However, both adaptive signaling and oncogenic (epidermal growth factor receptor (EGFR)-driven) signaling may induce PD-L1 upregulation in NSCLC. Nevertheless, the superiority of immune checkpoint inhibitors (ICIs) in advanced EGFR-mutant NSCLC is only moderate. ICIs appear to be well tolerated, but clinical activity for some advanced EGFR-mutant NSCLC patients has only been observed in a small proportion of trials. Hence, there are still several open questions about PD-L1 axis inhibitors in patients with NSCLC whose tumors harbor EGFR mutations, such as the effect of EGFR tyrosine kinase inhibitors (TKIs) or EGFR mutations in the tumor microenvironment (TME). Finding the answers to these questions requires ongoing trials and preclinical studies to identify the mechanisms explaining this possible increased susceptibility and to identify prognostic molecular and clinical markers that may predict benefits with PD-1 axis inhibition in this specific NSCLC subpopulation. The presence of multiple mechanisms, including dynamic immune TME profiles, changes in PD-L1 expression and low tumor mutational burdens, may explain the conflicting data regarding the correlation between PD-L1 axis inhibitors and EGFR mutation status. We conducted a review of this currently controversial topic in an attempt to aid in the decision-making process.
Keywords: Anti-PD-1/PD-L1 treatment; EGFR mutations; Immunotherapy; Non-small cell lung cancer; Tumor microenvironment.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Torre LA, Siegel RL, Jemal A. Lung cancer statistics. In Lung cancer and personalized medicine. Cham: Springer; 2016. pp. 1–19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

