Glucocorticoid-sensitive ventral hippocampal-orbitofrontal cortical connections support goal-directed action - Curt Richter Award Paper 2019
- PMID: 31526526
- PMCID: PMC6859207
- DOI: 10.1016/j.psyneuen.2019.104436
Glucocorticoid-sensitive ventral hippocampal-orbitofrontal cortical connections support goal-directed action - Curt Richter Award Paper 2019
Abstract
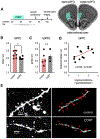
In an ever-changing and often ambiguous environment, organisms must use previously learned associations between antecedents and outcomes to predict future associations and make optimal choices. Chronic stress can impair one's ability to flexibly adjust behaviors when environmental contingencies change, particularly in cases of early-life stress. In mice, exposure to elevated levels of the primary stress hormone, corticosterone (CORT), during early adolescence is sufficient to impair response-outcome decision making later in life, biasing response strategies towards inflexible habits. Nevertheless, neurobiological mechanisms are still being defined. Here, we report that exposure to excess CORT in adolescence causes a loss of dendritic spines on excitatory pyramidal neurons in the lateral, but not medial, orbital prefrontal cortex (loPFC) of mice, and spine loss correlates with the severity of habit biases in adulthood. Excess CORT also reduces the presence of ventral hippocampal (vHC) axon terminals in the loPFC. To identify functional consequences, we inactivated vHC→loPFC projections in typical healthy mice during a period when mice must update response-outcome expectations to optimally acquire food reinforcers. Inactivation impaired the animals' subsequent ability to sustainably choose actions based on likely outcomes, causing them to defer to habit-based response strategies. Thus, vHC→loPFC projections are necessary for response-outcome expectancy updating and a target of excess glucocorticoids during early-life development. Their degradation is likely involved in long-term biases towards habit-based behaviors following glucocorticoid excess in adolescence.
Keywords: Action-outcome; Contingency degradation; DREADDs; Habit; Orbital frontal; Response-outcome.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
None.
Figures
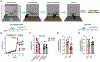
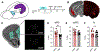

References
-
- Carr CP, Martins CM, Stingel AM, Lemgruber VB, Juruena MF, 2013. The role of early life stress in adult psychiatric disorders: a systematic review according to childhood trauma subtypes. J. Nerv. Ment. Dis 201, 1007–1020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

