High levels of serum hyaluronan is an early predictor of dengue warning signs and perturbs vascular integrity
- PMID: 31526718
- PMCID: PMC6838418
- DOI: 10.1016/j.ebiom.2019.09.014
High levels of serum hyaluronan is an early predictor of dengue warning signs and perturbs vascular integrity
Abstract
Background: A main pathological feature of severe dengue virus infection is endothelial hyper-permeability. The dengue virus nonstructural protein 1 (NS1) has been implicated in the vascular leakage that characterizes severe dengue virus infection, however, the molecular mechanisms involved are not known.
Methods: A cohort of 250 dengue patients has been followed from the onset of symptoms to the recovery phase. Serum hyaluronan levels and several other clinical parameters were recorded. The effect of NS1 treatment of cultured fibroblasts and endothelial cells on the expressions of hyaluronan synthetic and catabolic enzymes and the hyaluronan receptor CD44, were determined, as have the effects on the formation of hyaluronan-rich matrices and endothelial permeability.
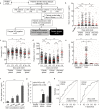
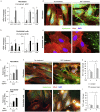
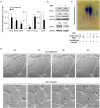
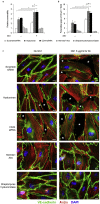
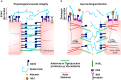
Findings: Elevated serum hyaluronan levels (≥70 ng/ml) during early infection was found to be an independent predictor for occurrence of warning signs, and thus severe dengue fever. High circulating levels of the viral protein NS1, indicative of disease severity, correlated with high concentrations of serum hyaluronan. NS1 exposure decreased the expression of CD44 in differentiating endothelial cells impairing the integrity of vessel-like structures, and promoted the synthesis of hyaluronan in dermal fibroblasts and endothelial cells in synergy with dengue-induced pro-inflammatory mediators. Deposited hyaluronan-rich matrices around cells cultured in vitro recruited CD44-expressing macrophage-like cells, suggesting a mechanism for enhancement of inflammation. In cultured endothelial cells, perturbed hyaluronan-CD44 interactions enhanced endothelial permeability through modulation of VE-cadherin and cytoskeleton re-organization, and exacerbated the NS1-induced disruption of endothelial integrity.
Interpretation: Pharmacological targeting of hyaluronan biosynthesis and/or its CD44-mediated signaling may limit the life-threatening vascular leakiness during moderate-to-severe dengue virus infection. FUND: This work was supported in part by grants from the Swedish Cancer Society (2018/337; 2016/445), the Swedish Research Council (2015-02757), the Ludwig Institute for Cancer Research, Uppsala University, the Ministry of Science and Technology, Taiwan (106-2314-B-037-088- and 106-2915-I-037-501-), Kaohsiung Medical University Hospital (KMUH103-3 T05) and Academy of Finland. The funders played no role in the design, interpretation or writing of the manuscript.
Keywords: CD44; Cytokines; Dengue; HAS2; HYAL2; Hyaluronan; TGFbeta; VE-cadherin; Vascular leakage.
Copyright © 2019. Published by Elsevier B.V.
Conflict of interest statement
CYL, YHC and PH have submitted a patent application based on the findings in this manuscript in United states (US 15/881,350) and EU (EP 18155997.2). CYL, YHC and PH are the inventors but not owner of a patent proved in Taiwan (TW I651535). The other authors have declared that no conflict of interest exists.
Figures


References
-
- Takahashi Y., Li L., Kamiryo M., Asteriou T., Moustakas A., Yamashita H. Hyaluronan fragments induce endothelial cell differentiation in a CD44- and CXCL1/GRO1-dependent manner. J Biol Chem. 2005;280(25):24195–24204. - PubMed
-
- West D.C., Hampson I.N., Arnold F., Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985;228:1324–1326. - PubMed
-
- Laurent T.C., Hyaluronan Fraser J.R.E. FASEB J. 1992;6:2397–2404. - PubMed
-
- Heldin P., Lin C.Y., Kolliopoulos C., Chen Y.H., Skandalis S.S. Regulation of hyaluronan biosynthesis and clinical impact of excessive hyaluronan production. Matrix Biol. 2019;78-79:100–117. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

