Nitrite pharmacokinetics, safety and efficacy after experimental ventricular fibrillation cardiac arrest
- PMID: 31526855
- PMCID: PMC6957908
- DOI: 10.1016/j.niox.2019.09.003
Nitrite pharmacokinetics, safety and efficacy after experimental ventricular fibrillation cardiac arrest
Abstract
Introduction: Besides therapeutic hypothermia or targeted temperature management no novel therapies have been developed to improve outcomes of patients after cardiac arrest (CA). Recent studies suggest that nitrite reduces neurological damage after asphyxial CA. Nitrite is also implicated as a new mediator of remote post conditioning produced by tourniquet inflation-deflation, which is under active investigation in CA. However, little is known about brain penetration or pharmacokinetics (PK). Therefore, to define the optimal use of this agent, studies on the PK of nitrite in experimental ventricular fibrillation (VF) are needed. We tested the hypothesis that nitrite administered after resuscitation from VF is detectable in cerebrospinal fluid (CSF), brain and other organ tissues, produces no adverse hemodynamic effects, and improves neurologic outcome in rats.
Methods: After return of spontaneous circulation (ROSC) of 5 min untreated VF, adult male Sprague-Dawley rats were given intravenous nitrite (8 μM, 0.13 mg/kg) or placebo as a 5 min infusion beginning at 5 min after CA. Additionally, sham groups with and without nitrite treatment were also studied. Whole blood nitrite levels were serially measured. After 15 min, CSF, brain, heart and liver tissue were collected. In a second series, using a randomized and blinded treatment protocol, rats were treated with nitrite or placebo after arrest. Neurological deficit scoring (NDS) was performed daily and eight days after resuscitation, fear conditioning testing (FCT) and brain histology were assessed.
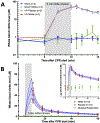
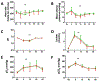
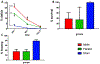
Results: In an initial series of experiments, rats (n = 21) were randomized to 4 groups: VF-CPR and nitrite therapy (n = 6), VF-CPR and placebo therapy (n = 5), sham (n = 5), or sham plus nitrite therapy (n = 5). Whole blood nitrite levels increased during drug infusion to 57.14 ± 10.82 μM at 11 min post-resuscitation time (1 min after dose completion) in the VF nitrite group vs. 0.94 ± 0.58 μM in the VF placebo group (p < 0.001). There was a significant difference between the treatment and placebo groups in nitrite levels in blood between 7.5 and 15 min after CPR start and between groups with respect to nitrite levels in CSF, brain, heart and liver. In a second series (n = 25 including 5 shams), 19 out of 20 animals survived until day 8. However, NDS, FCT and brain histology did not show any statistically significant difference between groups.
Conclusions: Nitrite, administered early after ROSC from VF, was shown to cross the blood brain barrier after a 5 min VF cardiac arrest. We characterized the PK of intravenous nitrite administration after VF and were able to demonstrate nitrite safety in this feasibility study.
Keywords: Animals; Asphyxia; Cardiopulmonary resuscitation; Heart arrest; Laboratory; Ventricular fibrillation.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

References
-
- Members WG, Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, Heart Disease and Stroke Statistics—2012 Update: A Report From the American Heart Association, Circulation 125 (2012) e2–e220. - PMC - PubMed
-
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K, Treatment of Comatose Survivors of Out-of-Hospital Cardiac Arrest with Induced Hypothermia, New Engl J Med 2002, pp. 557–563. - PubMed
-
- T. Hypothermia after Cardiac Arrest Study Group, Mild Therapeutic Hypothermia to Improve the Neurologic Outcome after Cardiac Arrest, New Engl J Med 2002, pp. 549–556. - PubMed
-
- Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M, Pellis T, Stammet P, Wanscher M, Wise MP, Åneman A, Al-Subaie N, Boesgaard S, Bro-Jeppesen J, Brunetti I, Bugge JF, Hingston CD, Juffermans NP, Koopmans M, Køber L, Langørgen J, Lilja G, Møller JE, Rundgren M, Rylander C, Smid O, Werer C, Winkel P, Friberg H, Targeted Temperature Management at 33°C versus 36°C after Cardiac Arrest, New England Journal of Medicine 369 (2013) 2197–2206. - PubMed
-
- Winther-Jensen M, Pellis T, Kuiper M, Koopmans M, Hassager C, Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Friberg H, Gasche Y, Horn J, Hovdenes J, Stammet P, Wanscher M, Wise MP, Åneman A, Kjaergaard J, Mortality and neurological outcome in the elderly after target temperature management for out-of-hospital cardiac arrest, Resuscitation 91 (2015) 92–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

