βγ-Crystallination Endows a Novel Bacterial Glycoside Hydrolase 64 with Ca2+-Dependent Activity Modulation
- PMID: 31527113
- PMCID: PMC6832075
- DOI: 10.1128/JB.00392-19
βγ-Crystallination Endows a Novel Bacterial Glycoside Hydrolase 64 with Ca2+-Dependent Activity Modulation
Abstract
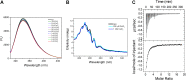
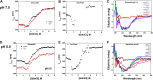
The prokaryotic βγ-crystallins are a large group of uncharacterized domains with Ca2+-binding motifs. We have observed that a vast number of these domains are found appended to other domains, in particular, the carbohydrate-active enzyme (CAZy) domains. To elucidate the functional significance of these prospective Ca2+ sensors in bacteria and this widespread domain association, we have studied one typical example from Clostridium beijerinckii, a bacterium known for its ability to produce acetone, butanol, and ethanol through fermentation of several carbohydrates. This novel glycoside hydrolase of family 64 (GH64), which we named glucanallin, is composed of a βγ-crystallin domain, a GH64 domain, and a carbohydrate-binding module 56 (CBM56). The substrates of GH64, β-1,3-glucans, are the targets for industrial biofuel production due to their plenitude. We have examined the Ca2+-binding properties of this protein, assayed its enzymatic activity, and analyzed the structural features of the β-1,3-glucanase domain through its high-resolution crystal structure. The reaction products resulting from the enzyme reaction of glucanallin reinforce the mixed nature of GH64 enzymes, in contrast to the prevailing notion of them being an exotype. Upon disabling Ca2+ binding and comparing different domain combinations, we demonstrate that the βγ-crystallin domain in glucanallin acts as a Ca2+ sensor and enhances the glycolytic activity of glucanallin through Ca2+ binding. We also compare the structural peculiarities of this new member of the GH64 family to two previously studied members.IMPORTANCE We have biochemically and structurally characterized a novel glucanase from the less studied GH64 family in a bacterium significant for fermentation of carbohydrates into biofuels. This enzyme displays a peculiar property of being distally modulated by Ca2+ via assistance from a neighboring βγ-crystallin domain, likely through changes in the domain interface. In addition, this enzyme is found to be optimized for functioning in an acidic environment, which is in line with the possibility of its involvement in biofuel production. Multiple occurrences of a similar domain architecture suggest that such a "βγ-crystallination"-mediated Ca2+ sensitivity may be widespread among bacterial proteins.
Keywords: Ca2+ binding; calcium binding; calcium-induced activity; crystal structure; glucanase; glycoside hydrolase; βγ-crystallins.
Copyright © 2019 American Society for Microbiology.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

