The huntingtin inclusion is a dynamic phase-separated compartment
- PMID: 31527136
- PMCID: PMC6749095
- DOI: 10.26508/lsa.201900489
The huntingtin inclusion is a dynamic phase-separated compartment
Abstract
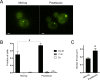
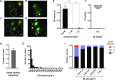
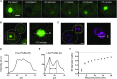
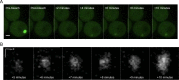
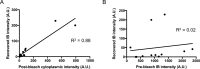
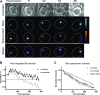
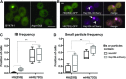
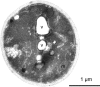
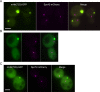
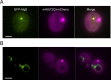
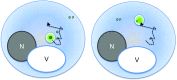
Inclusions of disordered protein are a characteristic feature of most neurodegenerative diseases, including Huntington's disease. Huntington's disease is caused by expansion of a polyglutamine tract in the huntingtin protein; mutant huntingtin protein (mHtt) is unstable and accumulates in large intracellular inclusions both in affected individuals and when expressed in eukaryotic cells. Using mHtt-GFP expressed in Saccharomyces cerevisiae, we find that mHtt-GFP inclusions are dynamic, mobile, gel-like structures that concentrate mHtt together with the disaggregase Hsp104. Although inclusions may associate with the vacuolar membrane, the association is reversible and we find that inclusions of mHtt in S. cerevisiae are not taken up by the vacuole or other organelles. Instead, a pulse-chase study using photoconverted mHtt-mEos2 revealed that mHtt is directly and continuously removed from the inclusion body. In addition to mobile inclusions, we also imaged and tracked the movements of small particles of mHtt-GFP and determine that they move randomly. These observations suggest that inclusions may grow through the collision and coalescence of small aggregative particles.
© 2019 Aktar et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Becher MW, Kotzuk JA, Sharp AH, Davies SW, Bates GP, Price DL, Ross CA (1998) Intranuclear neuronal inclusions in Huntington’s disease and dentatorubral and pallidoluysian atrophy: Correlation between the density of inclusions and IT15 CAG triplet repeat length. Neurobiol Dis 4: 387–397. 10.1006/nbdi.1998.0168 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
