Investigation of Metal-Organic Framework-5 (MOF-5) as an Antitumor Drug Oridonin Sustained Release Carrier
- PMID: 31527488
- PMCID: PMC6767262
- DOI: 10.3390/molecules24183369
Investigation of Metal-Organic Framework-5 (MOF-5) as an Antitumor Drug Oridonin Sustained Release Carrier
Abstract
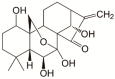
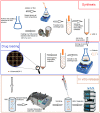
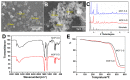
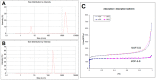
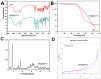
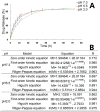
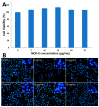
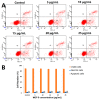
Oridonin (ORI) is a natural active ingredient with strong anticancer activity. But its clinical use is restricted due to its poor water solubility, short half-life, and low bioavailability. The aim of this study is to utilize the metal organic framework material MOF-5 to load ORI in order to improve its release characteristics and bioavailability. Herein, MOF-5 was synthesized by the solvothermal method and direct addition method, and characterized by Scanning Electron Microscopy (SEM), X-Ray Diffraction (XRD), Fourier Transform Infrared Spectrometer (FTIR), Thermogravimetric Analysis (TG), Brunauer-Emmett-Teller (BET), and Dynamic Light Scattering (DLS), respectively. MOF-5 prepared by the optimal synthesis method was selected for drug-loading and in vitro release experiments. HepG2 cells were model cells. MTT assay, 4',6-diamidino-2-phenylindole (DAPI) staining and Annexin V/PI assay were used to detect the biological safety of blank carriers and the anticancer activity of drug-loaded materials. The results showed that nano-MOF-5 prepared by the direct addition method had complete structure, uniform size and good biocompatibility, and was suitable as an ORI carrier. The drug loading of ORI@MOF-5 was 52.86% ± 0.59%. The sustained release effect was reliable, and the cumulative release rate was about 87% in 60 h. ORI@MOF-5 had significant cytotoxicity (IC50:22.99 μg/mL) and apoptosis effect on HepG2 cells. ORI@MOF-5 is hopeful to become a new anticancer sustained release preparation. MOF-5 has significant potential as a drug carrier material.
Keywords: Antitumor; HepG2 cells; MOF-5; Oridonin; sustained release.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Biocompatible Fe-Based Micropore Metal-Organic Frameworks as Sustained-Release Anticancer Drug Carriers.Molecules. 2018 Sep 28;23(10):2490. doi: 10.3390/molecules23102490. Molecules. 2018. PMID: 30274195 Free PMC article.
-
Synthesis and Application of a pH-Responsive Functional Metal-Organic Framework: In Vitro Investigation for Delivery of Oridonin in Cancer Therapy.Molecules. 2024 Jun 4;29(11):2643. doi: 10.3390/molecules29112643. Molecules. 2024. PMID: 38893518 Free PMC article.
-
Biodegradable self-assembled MPEG-PCL micelles for hydrophobic oridonin delivery in vitro.J Biomed Nanotechnol. 2012 Feb;8(1):80-9. doi: 10.1166/jbn.2012.1358. J Biomed Nanotechnol. 2012. PMID: 22515096
-
Recent Advances in Metal-Organic Frameworks as Anticancer Drug Delivery Systems: A Review.Anticancer Agents Med Chem. 2021;21(18):2487-2504. doi: 10.2174/1871520621666210119093844. Anticancer Agents Med Chem. 2021. PMID: 33463479 Review.
-
Solubility and Bioavailability Enhancement of Oridonin: A Review.Molecules. 2020 Jan 14;25(2):332. doi: 10.3390/molecules25020332. Molecules. 2020. PMID: 31947574 Free PMC article. Review.
Cited by
-
Multifaceted Applications of Zerumbone-Loaded Metal-Organic Framework-5: Anticancer, Antibacterial, Antifungal, DNA-Binding, and Free Radical Scavenging Potentials.Molecules. 2025 Jul 11;30(14):2936. doi: 10.3390/molecules30142936. Molecules. 2025. PMID: 40733202 Free PMC article.
-
Comparative removal of hazardous cationic dyes by MOF-5 and modified graphene oxide.Sci Rep. 2022 Sep 12;12(1):15314. doi: 10.1038/s41598-022-19550-5. Sci Rep. 2022. PMID: 36097048 Free PMC article.
-
[Recent advances in the applications of metal-organic frameworks-based molecularly imprinted materials].Se Pu. 2023 Aug;41(8):651-661. doi: 10.3724/SP.J.1123.2023.03005. Se Pu. 2023. PMID: 37534552 Free PMC article. Chinese.
-
Current status of controlled onco-therapies based on metal organic frameworks.RSC Adv. 2024 Apr 19;14(18):12817-12828. doi: 10.1039/d4ra00375f. eCollection 2024 Apr 16. RSC Adv. 2024. PMID: 38645527 Free PMC article. Review.
-
Antiviral Activity of Oridonin Against Herpes Simplex Virus Type 1.Drug Des Devel Ther. 2022 Dec 20;16:4311-4323. doi: 10.2147/DDDT.S387885. eCollection 2022. Drug Des Devel Ther. 2022. PMID: 36573068 Free PMC article.
References
-
- Fitzmaurice C., Akinyemiju T.F., Al Lami F.H., Alam T., Alizadeh-Navaei R., Allen C., Alsharif U., Alvis-Guzman N., Amini E., Anderson B.O., et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: A systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4:1553–1568. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 7194289/Natural Science Foundation of Beijing Municipality
- 81703715/National Natural Science Foundation of China
- 2017000020124G295/Training Programme Foundation for the Beijing Municipal Excellent Talents
- 2018-JYB-XJQ005/Beijing University of Chinese Medicine Scientific Research Project for Distinguished Young Scholar
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

