Autophagy Modulation as a Treatment of Amyloid Diseases
- PMID: 31527516
- PMCID: PMC6766836
- DOI: 10.3390/molecules24183372
Autophagy Modulation as a Treatment of Amyloid Diseases
Abstract
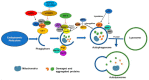
Amyloids are fibrous proteins aggregated into toxic forms that are implicated in several chronic disorders. More than 30 diseases show deposition of fibrous amyloid proteins associated with cell loss and degeneration in the affected tissues. Evidence demonstrates that amyloid diseases result from protein aggregation or impaired amyloid clearance, but the connection between amyloid accumulation and tissue degeneration is not clear. Common examples of amyloid diseases are Alzheimer's disease (AD), Parkinson's disease (PD) and tauopathies, which are the most common forms of neurodegenerative diseases, as well as polyglutamine disorders and certain peripheral metabolic diseases. In these diseases, increased accumulation of toxic amyloid proteins is suspected to be one of the main causative factors in the disease pathogenesis. It is therefore important to more clearly understand how these toxic amyloid proteins accumulate as this will aide in the development of more effective preventive and therapeutic strategies. Protein homeostasis, or proteostasis, is maintained by multiple cellular pathways-including protein synthesis, quality control, and clearance-which are collectively responsible for preventing protein misfolding or aggregation. Modulating protein degradation is a very complex but attractive treatment strategy used to remove amyloid and improve cell survival. This review will focus on autophagy, an important clearance pathway of amyloid proteins, and strategies for using it as a potential therapeutic target for amyloid diseases. The physiological role of autophagy in cells, pathways for its modulation, its connection with apoptosis, cell models and caveats in developing autophagy as a treatment and as a biomarker is discussed.
Keywords: Alzheimer’s disease; Huntington’s disease; Parkinson’s disease; Tau protein; amyloid; autophagy; beta amyloid; clearance; lysosome; polyglutamine; toxicity; α-synuclein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Verbeek M.M., Ruiter D.J., de Waal R.M. The role of amyloid in the pathogenesis of Alzheimer’s disease. Biol. Chem. 1997;378:937–950. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

