Energy-efficiency of Cardiomyocyte Stimulation with Rectangular Pulses
- PMID: 31527628
- PMCID: PMC6746869
- DOI: 10.1038/s41598-019-49791-w
Energy-efficiency of Cardiomyocyte Stimulation with Rectangular Pulses
Abstract
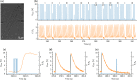
In cardiac pacemaker design, energy expenditure is an important issue. This work aims to explore whether varying stimulation pulse configuration is a viable optimization strategy for reducing energy consumption by the pacemaker. A single cardiomyocyte was used as an experimental model. Each cardiomyocyte was stimulated with different stimulation protocols using rectangular waveforms applied in varying number, in short succession. The amplitude, the width of each pulse, and the interval between consecutive pulses were modified. The application of multiple pulses in a short sequence led to a reduction of the threshold voltage required for stimulation when compared to a single pulse. However, none of the employed multi-pulse sequences reduced the overall energy expenditure of cell stimulation when compared to a single pulse stimulation. Among multiple pulse protocols, a combination of two short pulses (1 ms) separated with a short interval (0.5 ms) had the same energy requirements as a single short pulse (1 ms), but required the application of significantly less voltage. While increasing the number of consecutive pulses does not reduce the energy requirements of the pacemaker, the reduction in threshold voltage can be considered in practice if lower stimulation voltages are desired.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Elmgvist R, Landegren J, Pettersson S, Senning Å, William-Olsson G. Artificial pacemaker for treatment of adams-stokes syndrome and slow heart rate. Am. Heart J. 1963;65:731–748. doi: 10.1016/0002-8703(63)90239-4. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

