The reduction of miR146b-5p in monocytes and T cells could contribute to the immunopathogenesis of hepatitis C virus infection
- PMID: 31527804
- PMCID: PMC6746729
- DOI: 10.1038/s41598-019-49706-9
The reduction of miR146b-5p in monocytes and T cells could contribute to the immunopathogenesis of hepatitis C virus infection
Abstract
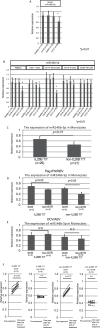
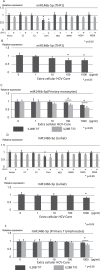
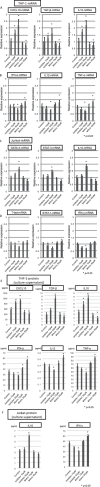
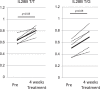
It has been reported that various kinds of miRNAs could affect the pathogenesis of hepatitis C virus infection. Recently, our group reported that deep-sequencing analysis was useful to detect disease-specific miRNAs. The aim of this study is to identify the HCV-specific miRNAs that could contribute to the immunopathogenesis of HCV by using clinical samples and in vitro analysis. Five miRNAs (hsa-miR181a-2-3p, hsa-miR-374a-3p, hsa-miR374a-5p, hsa-miR-204-5p and hsa-miR146b-5p) were shown to be significantly downregulated in CH-C by deep sequence analysis. The average ratio (PBMCs miRNAs/serum miRNAs) of hsa-miR146b-5p was highest among all the miRNAs. Moreover, serum hsa-miR146b-5p was significantly down-regulated in CH-C patients in comparison to CH-B patients and healthy subjects. The expression of hsa-miR146b-5p in CD3+ T cells and CD14+ monocytes of CH-C patients was significantly lower than that of the other groups. The hsa-miR146b-5p expression in CD14+ monocytes of SVR patients treated with Peg-IFN/RBV was significantly higher than in those of non-SVR patients treated with Peg IFN/RBV. However, the hsa-miR146b-5p expression in CD14+ monocytes of SVR patients treated with DCV and ASV was comparable to that in monocytes of non-SVR patients treated with DCV and ASV. Moreover, the expression levels of hsa-miR146b-5p in CD14+ monocytes were significantly increased after achieving SVR and 1(OH)Vitamin D3 treatment. Further, the expression of HCV-Core could suppress miR146b-5p expression in immune cells and affect the expression of various kinds of cytokines by affecting the NF-κB signaling. In conclusion, the reduction of miR146b-5p in monocytes and T cells could contribute to the immunopathogenesis of hepatitis C virus infection.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Aleman S, et al. A risk for hepatocellular carcinoma persists long-term after sustained virologic response in patients with hepatitis C-associated liver cirrhosis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013;57:230–236. doi: 10.1093/cid/cit234. - DOI - PubMed
-
- Chayama Kazuaki, Notsumata Kazuo, Kurosaki Masayuki, Sato Ken, Rodrigues Lino, Setze Carolyn, Badri Prajakta, Pilot-Matias Tami, Vilchez Regis A., Kumada Hiromitsu. Randomized trial of interferon- and ribavirin-free ombitasvir/paritaprevir/ritonavir in treatment-experienced hepatitis C virus-infected patients. Hepatology. 2015;61(5):1523–1532. doi: 10.1002/hep.27705. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

