Efficacy of tetravalent coryza vaccine against the challenge of Avibacterium paragallinarum serovars A and B isolates from Indonesia in chickens
- PMID: 31528020
- PMCID: PMC6702572
- DOI: 10.14202/vetworld.2019.972-977
Efficacy of tetravalent coryza vaccine against the challenge of Avibacterium paragallinarum serovars A and B isolates from Indonesia in chickens
Abstract
Aim: Infectious coryza is caused by Avibacterium paragallinarum. In Indonesia, this infection results in a 10%-40% decrease in egg production by laying hens. This study was conducted to determine the effectiveness of tetravalent coryza vaccine contained A. paragallinarum bacterin serovars A, B, C2, and C3; strain A-221, B-Spross, C2-Modesto, and C-3-Akko in layers based on antibody titer and clinical signs using a post-challenge test.
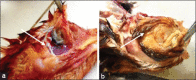
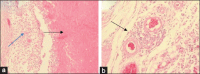
Materials and methods: Forty four-week-old Lohmanns strain chickens were used in this study. Forty chickens were divided into four groups for serological and challenge test: Group 1 (unvaccinated and challenged by A. paragallinarum serovar A), Group 2 (unvaccinated and challenged by A. paragallinarum serovar B), Group 3 (vaccinated and challenged by A. paragallinarum serovar A), and Group 4 (vaccinated and challenged by A. paragallinarum serovar B). Vaccination was done using the tetravalent vaccine in oil-emulsion adjuvant contained A. paragallinarum bacterin serovars A, B, C2, and C3; strain A-221, B-Spross, C2-Modesto, and C-3-Akko. Vaccination was performed at day 1 and booster was done at day 14. Blood serum was collected on days 0, 14, and 28 for the hemagglutination-hemagglutination inhibition (HI) test. The challenge test was given at day 29 through intranasal administration using A. paragallinarum serovars A-L2447 and B-L1710 approximately 6×108 CFU/mL. Clinical signs were observed for 14 days post-infection. At the end of the study, chickens were euthanized, and pathological features of the infraorbital sinus, facial skin, and trachea were recorded.
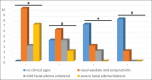
Results: Data analysis of antibody titers and pathological changes was performed descriptively, while clinical symptom scores were analyzed non-parametrically with the Mann-Whitney U-test using SPSS version 21. At days 14 and 28 post-vaccination, the antibody titer in Group 3 was 5 HI and 20 HI, respectively. However, the antibody titers in Group 4 at 28 days post-vaccination were 0 HI. Clinical observations, the vaccinated groups that were challenged with A. paragallinarum serovars A and B showed clinical symptoms on days 4 and 6 post-infection, namely mild unilateral facial edema and severe bilateral facial edema, respectively. Clinical signs in Groups 3 and 4 were less severe than in Groups 1 and 2 (p<0.05). Pathological examination findings supported clinical observations and serological testing.
Conclusion: Tetravalent coryza vaccine in chickens has efficacy to protect against the challenge test of A. paragallinarum serovars A and B isolated from Indonesia.
Keywords: Avibacterium paragallinarum; challenge test; clinical signs; serological test; vaccine.
Figures



References
-
- Anjaneya S.D, Singh K, Dhama V, Gowthaman Chawak M.M. Pathogenicity study of field isolates of Avibacterium paragallinarum in experimentally infected birds. Indian J. Vet. Pathol. 2013;37(1):13–17.
-
- Akhter S, Ali M, Das P.M, Hossain M.M. Isolation and identification of Avibacterium paragallinarum, the causal agent of infectious coryza (IC) from layer chickens in Bangladesh. J. Bangladesh Agric. Univ. 2013;11(1):87–96.
-
- Durairajan R, Sharma M, Murugan M.S. Detection of Avibacterium paragallinarum in commercial poultry and their antibiogram. Tamil Nadu J. Vet. Anim. Sci. Res. 2013;9(5):332–337.
-
- Patil V.V, Mishra D.N, Mane D.V. Isolation, characterization and serological study of Avibacterium paragallinarum field isolates from Indian poultry. J. Anim. Poult. Sci. 2016;5(1):13–20.
-
- Blackall P.J, Soriano-Vargas E. Infectious coryza and related bacterial infections. In: Swayne D.E, Pattison M, Mc Mullin P.F, Bradbury J.M, editors. Disease of Poultry. 13th ed. Ch. 20. India: John Wiley and Sons; 2013. pp. 859–873.
LinkOut - more resources
Full Text Sources
Miscellaneous
