Detection of lumpy skin disease virus in cattle using real-time polymerase chain reaction and serological diagnostic assays in different governorates in Egypt in 2017
- PMID: 31528038
- PMCID: PMC6702561
- DOI: 10.14202/vetworld.2019.1093-1100
Detection of lumpy skin disease virus in cattle using real-time polymerase chain reaction and serological diagnostic assays in different governorates in Egypt in 2017
Abstract
Background and aim: Lumpy skin disease (LSD), is a highly infectious viral disease of cattle, caused by LSD virus (LSDV) which belongs to the genus Capripoxvirus of family Poxviridae. In the summer of 2017, skin lesions suggestive of LSD were observed in cattle at several governorates in Egypt. This study aimed to detect LSDV in cattle specimens using rapid serological and molecular diagnostic assays.
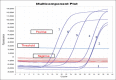
Materials and methods: A total of 46 skin biopsies and uncoagulated blood samples were collected from cattle with LSD suggestive clinical signs, as well as 290 coagulated whole blood samples from cattle without skin lesion in different governorates in Egypt during the summer of 2017. Skin biopsies were used for virus isolation from the chorioallantoic membrane of 11-day-old specific pathogen-free embryonated chicken eggs (SPF-ECEs). LSDV was identified using conventional polymerase chain reaction (PCR), real-time PCR (RT-PCR), and fluorescent antibody technique (FAT) with specific hyperimmune serum against LSDV. Cattle sera were examined using indirect FAT (IFAT) and indirect enzyme-linked immunosorbent assay (ELISA).
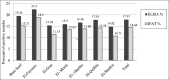
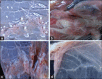
Results: Skin nodules and sitfast lesions were significant clinical signs observed in all LSD suspect cattle. SPF-ECEs, from which positive isolations were made and it showed characteristic inflammatory and focal white pock lesions. The isolated viruses were identified as LSDV by FAT, conventional gel-based PCR, and RT-PCR. Among the skin biopsies and corresponding blood samples, LSDV-positive samples percentage were 39.13 and 36.95 by RT-PCR, followed 34.78 and 28.26 by conventional PCR and then 32.6 and 26.8 by FAT, respectively. The total positive percentage of LSDV antibody detected in cattle serum samples were 17.93 and 14.48 by indirect ELISA and IFAT.
Conclusion: LSDV was detected and identified in skin biopsies and corresponding blood samples of naturally infected cattle, more LSDV-positive samples were detected by RT-PCR, followed by conventional PCR and then FAT. The indirect ELISA detected more antibody-positive samples than the IFAT from cattle serum samples. The RT-PCR assay is simple, sensitive, rapid, and reliable for the detection of LSDV in blood and skin nodule biopsies of suspected cattle.
Keywords: Poxviridae; enzyme-linked immunosorbent assay; indirect fluorescent antibody technique; lumpy skin disease; polymerase chain reaction; real-time polymerase chain reaction.
Figures


References
-
- Mathews R.E.F. Classification and nomenclature of viruses. Intervirology. 1982;17(1-3):191–199. - PubMed
-
- Bhanuprakash V, Indrani B.K, Hosamani M, Singh R.K. The current status of sheep pox disease. Comp. Immunol. Microbiol. Infect. Dis. 2006;29(1):27–60. - PubMed
-
- El-Nahas E, El-Habbaa A, El-Bagoury G, Radwan M.E. Isolation and identification of lumpy skin disease virus from naturally infected buffaloes at Kaluobia, Egypt. Glob. Vet. 2011;7(3):234–237.
-
- Birhanu H, Gezahign A, Nuru S.D. Epidemiology, economic importance and control techniques of lumpy skin diseases. Anim. Vet. Sci. 2015;3(2):58–66.
LinkOut - more resources
Full Text Sources
