Effect of mild moxibustion on intestinal microbiota and NLRP6 inflammasome signaling in rats with post-inflammatory irritable bowel syndrome
- PMID: 31528095
- PMCID: PMC6718040
- DOI: 10.3748/wjg.v25.i32.4696
Effect of mild moxibustion on intestinal microbiota and NLRP6 inflammasome signaling in rats with post-inflammatory irritable bowel syndrome
Abstract
Background: About one-third of refractory irritable bowel syndrome (IBS) cases are caused by gastrointestinal (GI) infection/inflammation, known as post-infectious/post-inflammatory IBS (PI-IBS). Although it is known that intestinal microbiota and host NOD-like receptor family pyrin domain containing 6 (NLRP6) inflammsome signaling are closely related to PI-IBS and moxibustion has a therapeutic effect on PI-IBS, whether moxibustion regulates the intestinal flora and host NLRP6 events in PI-IBS remains unclear.
Aim: To examine the regulatory effect of moxibustion on intestinal microbiota and host NLRP6 inflammatory signaling in PI-IBS.
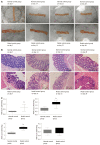
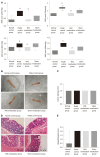
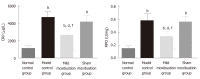
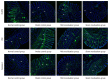
Methods: Sprague-Dawley rats were divided into a normal control group, a model control group, a mild moxibustion group, and a sham mild moxibustion group. PI-IBS rats in the mild moxibustion group were treated with moxibusiton at bilateral Tianshu (ST 25) and Zusanli (ST36) for 7 consecutive days for 10 min each time. The sham group rats were given the same treatment as the mild moxibustion group except the moxa stick was not ignited. Abdominal withdrawal reflex (AWR) score was measured to assess the visceral sensitivity, and colon histopathology and ultrastructure, colonic myeloperoxidase (MPO) activity, and serum C-reactive protein (CRP) level were measured to evaluate low-grade colonic inflammation in rats. The relative abundance of selected intestinal bacteria in rat feces was detected by 16S rDNA PCR and the NLRP6 inflammsome signaling in the colon was detected by immunofluorescence, qRT-PCR, and Western blot.
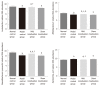
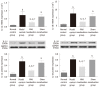
Results: The AWR score was significantly decreased and the low-grade intestinal inflammation reflected by serum CRP and colonic MPO levels was inhibited in the mild moxibustion group compared with the sham group. Mild moxibustion remarkably increased the relative DNA abundances of Lactobacillus, Bifidobacterium, and Faecalibacterium prausnitzii but decreased that of Escherichia coli in the gut of PI-IBS rats. Additionally, mild moxibustion induced mRNA and protein expression of intestine lectin 1 but inhibited the expression of IL-1β, IL-18, and resistance-like molecule β by promoting the NLRP6 and reducing the mRNA and protein expression of apoptosis-associated speck-like protein containing CARD (ASC) and cysteinyl-aspartate-specific proteinase 1 (Caspase-1). The relative DNA abundances of Lactobacillus, Bifidobacteria, Faecalibacterium prausnitzii, and Escherichia coli in each group were correlated with the mRNA and protein expression of NLRP6, ASC, and Caspase-1 in the colon.
Conclusion: These findings indicated that mild moxibustion can relieve low-grade GI inflammation and alleviate visceral hypersensitivity in PI-IBS by regulating intestinal microbes and controlling NLRP6 inflammasome signaling.
Keywords: Intestinal inflammation; Intestinal microbes; Moxibustion; NLRP6 inflammasome; Post-inflammation irritable bowel syndrome; Traditional Chinese medicine; Visceral hypersensitivity.
Conflict of interest statement
Conflict-of-interest statement: The authors declare no conflict of interest related to this study.
Figures



References
-
- Liu J, Hou X. A review of the irritable bowel syndrome investigation on epidemiology, pathogenesis and pathophysiology in China. J Gastroenterol Hepatol. 2011;26:88–93. - PubMed
-
- Barbara G, Cremon C, Pallotti F, De Giorgio R, Stanghellini V, Corinaldesi R. Postinfectious irritable bowel syndrome. J Pediatr Gastroenterol Nutr. 2009;48:S95–S97. - PubMed
-
- Wang H, Chang L. The Walkerton outbreak revisited at year 8: Predictors, prevalence, and prognosis of postinfectious irritable bowel syndrome. Gastroenterology. 2011;140:726–8; discussion 728-9. - PubMed
-
- Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979–1988. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

