Aluminum Phosphate Vaccine Adjuvant: Analysis of Composition and Size Using Off-Line and In-Line Tools
- PMID: 31528298
- PMCID: PMC6739432
- DOI: 10.1016/j.csbj.2019.08.003
Aluminum Phosphate Vaccine Adjuvant: Analysis of Composition and Size Using Off-Line and In-Line Tools
Abstract
Purpose: Aluminum-based adjuvants including aluminum phosphate (AlPO4) are commonly used in many human vaccines to enhance immune response. The interaction between the antigen and adjuvant, including the physical adsorption of antigen, may play a role in vaccine immunogenicity and is a useful marker of vaccine product quality and consistency. Thus, it is important to study the physicochemical properties of AlPO4, such as particle size and chemical composition. Control of the vaccine adjuvant throughout the manufacturing process, including raw materials and the intermediate and final product stages, can be effectively achieved through monitoring of such key product attributes to help ensure product quality.
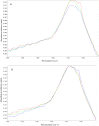
Methods: This study focuses on the compositional analysis of AlPO4 adjuvant at the intermediate and final manufacturing stages using the off-line methods Fourier-Transform Infrared (FTIR) and Raman spectroscopy, X-ray Photoelectron Spectroscopy (XPS), and the in-line method Attenuated Total Reflectance (ATR). Particle size distribution of AlPO4 was measured off-line using Laser diffraction (LD) and in-line using Focused Beam Reflectance Measurement (FBRM®).
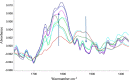
Results: There was no observable difference in size distribution between the intermediate and final stage AlPO4 by off-line and in-line analysis, in both small- or large-scale production samples. Consistent peak shifts were observed in off-line and in-line infrared (IR) spectroscopy as well as off-line XPS for both small- and large-scale AlPO4 manufacturing runs. Additionally, IR spectroscopy and FBRM® for size distribution were used as in-line process analytical technology (PAT) to monitor reaction progress in real-time during small-scale AlPO4 manufacturing from raw materials. The small-scale adsorption process of a model protein antigen (Tetanus toxoid) to AlPO4 adjuvant was also monitored by in-line ReactIR probe.
Conclusion: This study demonstrated that in-line PAT can be used to monitor particle size and chemical composition for the various stages of adjuvant manufacturing from raw materials through intermediate to final adjuvant product stage. Similar approaches can be utilized to help assess lot-to-lot consistency during adjuvant manufacturing and vaccine product development. Moreover, the use of in-line PAT is highly conductive to advanced manufacturing strategies such as real-time product release testing and automated processes of the future.
Keywords: Aluminum phosphate adjuvant (AlPO4); Focused beam reflectance measurement (FBRM®); Fourier transform infrared spectroscopy (FTIR); Laser diffraction (LD); Particle size distribution; Process analytical technology (PAT); Raman spectroscopy; X-ray photoelectron spectroscopy (XPS).
Conflict of interest statement
Carmen Mei, Sasmit Deshmukh, Bruce Carpick, Matthew Balmer, Daniel Chapman, Nicole Lazaris, Liliana Sampaleanu, and Marina Kirkitadze are employees of Sanofi Pasteur. Jim Cronin, Shuxin Cong, Ulrich Schacht, and Katherine Drolet-Vives, Moriam Ore and Sylvie Morin have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Figures






References
-
- Hem S.L., Hogenesch H. Relationship between physical and chemical properties of aluminum-containing adjuvants and immunopotentiation. Expert Rev Vaccines. 2007;6(5):685–698. - PubMed
-
- Gupta R.K. Aluminum compounds as vaccine adjuvants. Adv Drug Deliv Rev. 1998;32(3):155–172. - PubMed
-
- Redman T., O'Grady D. American Institute of Chemical Engineers; 2017. Improve products and processes with in-line particle analysis; pp. 47–55.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
