Peripheral sensory neurons govern development of the nervous system in bivalve larvae
- PMID: 31528326
- PMCID: PMC6743156
- DOI: 10.1186/s13227-019-0133-6
Peripheral sensory neurons govern development of the nervous system in bivalve larvae
Abstract
Recent findings regarding early lophotrochozoan development have altered the conventional model of neurogenesis and revealed that peripheral sensory elements play a key role in the initial organization of the larval nervous system. Here, we describe the main neurogenetic events in bivalve mollusks in comparison with other Lophotrochozoa, emphasizing a novel role for early neurons in establishing larval nervous systems and speculating about the morphogenetic function of the apical organ. We demonstrate that during bivalve development, peripheral sensory neurons utilizing various transmitters differentiate before the apical organ emerges. The first neurons and their neurites serve as a scaffold for the development of the nervous system. During veliger stage, cerebral, pleural, and visceral ganglia form along the lateral (visceral) nerve cords in anterior-to-posterior axis. The pedal ganglia and corresponding ventral (pedal) nerve cords develop much later, after larval settlement and metamorphosis. Pharmacological abolishment of the serotonin gradient within the larval body disrupts the navigation of "pioneer" axons resulting in malformation of the whole nervous system architecture. Comparative morphological data on neurogenetic events in bivalve mollusks shed new light on the origin of the nervous system, mechanisms of early axon navigation, and sequence of the tetraneurous nervous system formation. Furthermore, this information improves our understanding of the basic nervous system architecture in larval Bivalvia and Mollusca.
Keywords: Ganglia; Mollusk; Nerve cords; Neurotransmitters; Tetraneuralia.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures
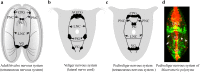
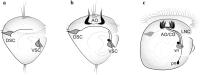
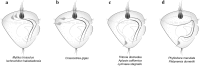
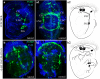

References
-
- Erdmann W. Nr. 5. Ober die Entwicklung und die Anatomie der ansatzreifen Larve von Ostrea edulis mit Bemerkungen iiber die Lebensgeschichte der Auster. Untersuchungen iiber die Lebensgeschichte der Auster; 1934. p. 1–25.
-
- Voronezhskaya EE, Nezlin LP, Odintsova NA, Plummer JT, Croll RP. Neuronal development in larval mussel Mytilus trossulus (Mollusca: Bivalvia) Zoomorphology. 2008;127:97–110. doi: 10.1007/s00435-007-0055-z. - DOI
-
- Wanninger A. Evolutionary developmental biology of invertebrates 2: lophotrochozoa spiralia. Wien: Springer; 2015.
Publication types
LinkOut - more resources
Full Text Sources

