Effect of Vaginal Mesh Hysteropexy vs Vaginal Hysterectomy With Uterosacral Ligament Suspension on Treatment Failure in Women With Uterovaginal Prolapse: A Randomized Clinical Trial
- PMID: 31529008
- PMCID: PMC6749543
- DOI: 10.1001/jama.2019.12812
Effect of Vaginal Mesh Hysteropexy vs Vaginal Hysterectomy With Uterosacral Ligament Suspension on Treatment Failure in Women With Uterovaginal Prolapse: A Randomized Clinical Trial
Erratum in
-
Error in Results Section.JAMA. 2021 Feb 16;325(7):696. doi: 10.1001/jama.2021.0535. JAMA. 2021. PMID: 33591329 Free PMC article. No abstract available.
Abstract
Importance: Vaginal hysterectomy with suture apical suspension is commonly performed for uterovaginal prolapse. Transvaginal mesh hysteropexy is an alternative option.
Objective: To compare the efficacy and adverse events of vaginal hysterectomy with suture apical suspension and transvaginal mesh hysteropexy.
Design, setting, participants: At 9 clinical sites in the US Pelvic Floor Disorders Network, 183 postmenopausal women with symptomatic uterovaginal prolapse were enrolled in a randomized superiority clinical trial between April 2013 and February 2015. The study was designed for primary analysis when the last randomized participant reached 3 years of follow-up in February 2018.
Interventions: Ninety-three women were randomized to undergo vaginal mesh hysteropexy and 90 were randomized to undergo vaginal hysterectomy with uterosacral ligament suspension.
Main outcomes and measures: The primary treatment failure composite outcome (re-treatment of prolapse, prolapse beyond the hymen, or prolapse symptoms) was evaluated with survival models. Secondary outcomes included operative outcomes and adverse events, and were evaluated with longitudinal models or contingency tables as appropriate.
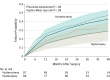
Results: A total of 183 participants (mean age, 66 years) were randomized, 175 were included in the trial, and 169 (97%) completed the 3-year follow-up. The primary outcome was not significantly different among women who underwent hysteropexy vs hysterectomy through 48 months (adjusted hazard ratio, 0.62 [95% CI, 0.38-1.02]; P = .06; 36-month adjusted failure incidence, 26% vs 38%). Mean (SD) operative time was lower in the hysteropexy group vs the hysterectomy group (111.5 [39.7] min vs 156.7 [43.9] min; difference, -45.2 [95% CI, -57.7 to -32.7]; P = <.001). Adverse events in the hysteropexy vs hysterectomy groups included mesh exposure (8% vs 0%), ureteral kinking managed intraoperatively (0% vs 7%), granulation tissue after 12 weeks (1% vs 11%), and suture exposure after 12 weeks (3% vs 21%).
Conclusions and relevance: Among women with symptomatic uterovaginal prolapse undergoing vaginal surgery, vaginal mesh hysteropexy compared with vaginal hysterectomy with uterosacral ligament suspension did not result in a significantly lower rate of the composite prolapse outcome after 3 years. However, imprecision in study results precludes a definitive conclusion, and further research is needed to assess whether vaginal mesh hysteropexy is more effective than vaginal hysterectomy with uterosacral ligament suspension.
Trial registration: ClinicalTrials.gov Identifier: NCT01802281.
Conflict of interest statement
Figures
Comment in
-
Pelvic Organ Prolapse: Reconsidering Treatment, Innovation, and Failure.JAMA. 2019 Sep 17;322(11):1047-1048. doi: 10.1001/jama.2019.12245. JAMA. 2019. PMID: 31528990 No abstract available.
-
Database Updates and Resulting Changes in Data in Study of Vaginal Mesh Hysteropexy vs Vaginal Hysterectomy.JAMA. 2021 Feb 16;325(7):695-696. doi: 10.1001/jama.2021.0477. JAMA. 2021. PMID: 33591341 No abstract available.
References
Publication types
MeSH terms
Associated data
Grants and funding
- U10 HD054215/HD/NICHD NIH HHS/United States
- U10 HD069025/HD/NICHD NIH HHS/United States
- U10 HD041261/HD/NICHD NIH HHS/United States
- U10 HD069010/HD/NICHD NIH HHS/United States
- U10 HD041267/HD/NICHD NIH HHS/United States
- UG1 HD069010/HD/NICHD NIH HHS/United States
- U10 HD069013/HD/NICHD NIH HHS/United States
- UG1 HD054214/HD/NICHD NIH HHS/United States
- U01 HD069031/HD/NICHD NIH HHS/United States
- UG1 HD054215/HD/NICHD NIH HHS/United States
- UG1 HD069006/HD/NICHD NIH HHS/United States
- U10 HD069006/HD/NICHD NIH HHS/United States
- UG1 HD041261/HD/NICHD NIH HHS/United States
- U24 HD069031/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

