N-Acenoacenes
- PMID: 31529726
- PMCID: PMC7687221
- DOI: 10.1002/chem.201903646
N-Acenoacenes
Abstract
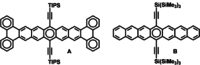
The syntheses of new, fourfold alkynylated tetraazaacenoacenes (tetraazaanthracenoanthracene, tetraazatetracenotetracene and tetraazapentacenopentacene) are reported. This novel heteroacenoacene motif exhibits surprisingly strong electronic coupling between its constituting diazaacene units.
Keywords: 2D acenes; aromaticity; azaacenes; electronic coupling; semiconductors.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- None
-
- Anthony J. E., Chem. Rev. 2006, 106, 5028–5048; - PubMed
-
- Anthony J. E., Angew. Chem. Int. Ed. 2008, 47, 452–483; - PubMed
- Angew. Chem. 2008, 120, 460–492;
-
- Bunz U. H. F., Acc. Chem. Res. 2015, 48, 1676–1686; - PubMed
-
- Bunz U. H. F., Engelhart J. U., Lindner B. D., Schaffroth M., Angew. Chem. Int. Ed. 2013, 52, 3810–3821; - PubMed
- Angew. Chem. 2013, 125, 3898–3910;
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

