Circadian Function in Multiple Cell Types Is Necessary for Proper Timing of the Preovulatory LH Surge
- PMID: 31530063
- PMCID: PMC9206841
- DOI: 10.1177/0748730419873511
Circadian Function in Multiple Cell Types Is Necessary for Proper Timing of the Preovulatory LH Surge
Abstract
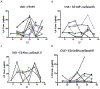
The timing of the preovulatory surge of luteinizing hormone (LH), which occurs on the evening of proestrus in female mice, is determined by the circadian system. The identity of cells that control the phase of the LH surge is unclear: evidence supports a role of arginine vasopressin (AVP) cells of the suprachiasmatic nucleus (SCN), but it is not known whether vasopressinergic neurons are necessary or sufficient to account for circadian control of ovulation. Among other cell types, evidence also suggests important roles of circadian function of kisspeptin cells of the anteroventral periventricular nucleus (AvPV) and gonadotropin-releasing hormone (GnRH) neurons of the preoptic area (POA), whose discharge is immediately responsible for the discharge of LH from the anterior pituitary. The present studies used an ovariectomized, estradiol-treated preparation to determine critical cell types whose clock function is critical to the timing of LH secretion. As expected, the LH surge occurred at or shortly after ZT12 in control mice. In further confirmation of circadian control, the surge was advanced by 2 h in tau mutant animals. The timing of the surge was altered to varying degrees by conditional deletion of Bmal1 in AVPCre, KissCreBAC, and GnRHCreBAC mice. Excision of the mutant Cnsk1e (tau) allele in AVP neurons resulted in a reversion of the surge to the ZT12. Conditional deletion of Bmal1 in Kiss1 or GnRH neurons had no noticeable effect on locomotor rhythms, but targeting of AVP neurons produced variable effects on circadian period that did not always correspond to changes in the phase of LH secretion. The results indicate that circadian function in multiple cell types is necessary for proper timing of the LH surge.
Keywords: Bmal1; GnRH; circadian; kisspeptin; luteinizing hormone; vasopressin.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
The author has no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
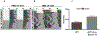

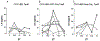
References
-
- Alexander MJ, Clifton DK, and Steiner RA (1985) Vasoactive intestinal polypeptide effects a central inhibition of pulsatile luteinizing hormone secretion in ovariectomized rats. Endocrinology 117:2134–2139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

