Blocking Notch signal pathway suppresses the activation of neurotoxic A1 astrocytes after spinal cord injury
- PMID: 31530090
- PMCID: PMC6791691
- DOI: 10.1080/15384101.2019.1667189
Blocking Notch signal pathway suppresses the activation of neurotoxic A1 astrocytes after spinal cord injury
Abstract
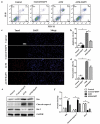
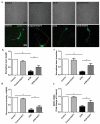
Spinal cord injury (SCI) is a catastrophic disease which has complicated pathogenesis including inflammation, oxidative stress and glial scar formation. Astrocytes are the most abundant cells in central nervous system and fulfill homeostatic functions. Recent studies have described a new reactive phenotype of astrocytes, A1, induced by inflammation, which may have negative effects in SCI. As the Notch signaling pathway has been linked to cell differentiation and inflammation, we aimed to investigate its potential role in the differentiation of astrocytes in SCI. Contusive SCI rat model showed elevated A1 astrocyte numbers at the damage site 28 days after SCI and the expression levels of Notch signaling and its downstream genes were upregulated parallelly. Western blotting, RT-qPCR and immunofluorescence revealed that blocking of Notch pathway using γ-secretase blocker (DAPT) suppressed the differentiation of A1 astrocytes. Flow cytometry, and TUNEL staining indicated that DAPT alleviated neuronal apoptosis and axonal damage caused by A1 astrocytes likely through the Notch-dependent release of pro-inflammatory factors. CO-IP and western blotting revealed an interaction between Notch pathway and signal transducer and activator of transcription 3 (Stat3), which played a vital role in differentiation of A1 astrocytes. We conclude that phenotypic transition of A1 astrocytes and their neurotoxity were controlled by the Notch-Stat3 axis and that Notch pathway in astrocytes may serve as a promising therapeutic target for SCI.
Keywords: A1 astrocytes; DAPT; Notch signaling; Spinal cord injury; Stat3.
Figures






References
-
- Ahuja CS, Wilson JR, Nori S, et al. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3:17018. - PubMed
-
- Jazayeri SB, Beygi S, Shokraneh F, et al. Incidence of traumatic spinal cord injury worldwide: a systematic review. Eur Spine J. 2015;24:905–918. - PubMed
-
- Pineau I, Lacroix S.. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol. 2007;500:267–285. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
