Evidence that tissue resident human enthesis γδT-cells can produce IL-17A independently of IL-23R transcript expression
- PMID: 31530557
- PMCID: PMC6837256
- DOI: 10.1136/annrheumdis-2019-215210
Evidence that tissue resident human enthesis γδT-cells can produce IL-17A independently of IL-23R transcript expression
Abstract
Objectives: Murine models of interleukin (IL)-23-driven spondyloarthritis (SpA) have demonstrated entheseal accumulation of γδT-cells which were responsible for the majority of local IL-17A production. However, IL-23 blockers are ineffective in axial inflammation in man. This study investigated γδT-cell subsets in the normal human enthesis to explore the biology of the IL-23/17 axis.
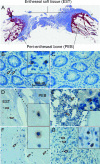
Methods: Human spinous processes entheseal soft tissue (EST) and peri-entheseal bone (PEB) were harvested during elective orthopaedic procedures. Entheseal γδT-cells were evaluated using immunohistochemistry and isolated and characterised using flow cytometry. RNA was isolated from γδT-cell subsets and analysed by qPCR. Entheseal γδT-cells were stimulated with phorbol 12-myristate 13-acetate (PMA) and ionomycin, anti-CD3/28 or IL-23 and IL-17A production was measured by high-sensitivity ELISA and qPCR.
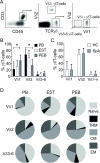
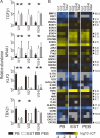
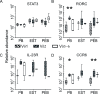
Results: Entheseal γδT-cells were confirmed immunohistochemically with Vδ1 and Vδ2 subsets that are cytometrically defined. Transcript profiles of both cell populations suggested tissue residency and immunomodulatory status. Entheseal Vδ2 cells expressed high relative abundance of IL-23/17-associated transcripts including IL-23R, RORC and CCR6, whereas the Vδ1 subset almost completely lacked detectable IL-23R transcript. Following PMA stimulation IL-17A was detectable in both Vδ1 and Vδ2 subsets, and following CD3/CD28 stimulation both subsets showed IL-17A and IL-17F transcripts with neither transcript being detectable in the Vδ1 subset following IL-23 stimulation.
Conclusion: Spinal entheseal Vδ1 and Vδ2 subsets are tissue resident cells with inducible IL-17A production with evidence that the Vδ1 subset does so independently of IL-23R expression.
Keywords: T cells; ankylosing spondylitis; psoriatic arthritis; spondyloarthritis.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

