Efficacy, Safety, and Tolerability of Oral Semaglutide Versus Placebo Added to Insulin With or Without Metformin in Patients With Type 2 Diabetes: The PIONEER 8 Trial
- PMID: 31530667
- PMCID: PMC7364672
- DOI: 10.2337/dc19-0898
Efficacy, Safety, and Tolerability of Oral Semaglutide Versus Placebo Added to Insulin With or Without Metformin in Patients With Type 2 Diabetes: The PIONEER 8 Trial
Abstract
Objective: To investigate the efficacy, safety, and tolerability of oral semaglutide added to insulin with or without metformin.
Research design and methods: Patients with type 2 diabetes uncontrolled on insulin with or without metformin were randomized to oral semaglutide 3 mg (N = 184), 7 mg (N = 182), or 14 mg (N = 181) or to placebo (N = 184) in a 52-week, double-blind trial. End points were change from baseline to week 26 in HbA1c (primary) and body weight (confirmatory secondary). Two estimands were defined: treatment policy (effect regardless of trial product discontinuation or rescue medication) and trial product (effect assuming trial product continuation without rescue medication) in randomized patients.
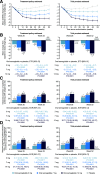
Results: Oral semaglutide was superior to placebo in reducing HbA1c (estimated treatment difference [ETD] -0.5% [95% CI -0.7, -0.3], -0.9% [-1.1, -0.7], and -1.2% [-1.4, -1.0] for 3, 7, and 14 mg, respectively; P < 0.001) and body weight (ETD -0.9 kg [95% CI -1.8, -0.0], -2.0 kg [-3.0, -1.0], and -3.3 kg [-4.2, -2.3]; P = 0.0392 for 3 mg, P ≤ 0.0001 for 7 and 14 mg) at week 26 (treatment policy estimand). Significantly greater dose-dependent HbA1c and body weight reductions versus placebo were achieved with oral semaglutide at weeks 26 and 52 (both estimands). The most frequent adverse event with oral semaglutide was nausea (11.4-23.2% of patients vs. 7.1% with placebo; mostly mild to moderate).
Conclusions: Oral semaglutide was superior to placebo in reducing HbA1c and body weight when added to insulin with or without metformin in patients with type 2 diabetes. The safety profile was consistent with other glucagon-like peptide 1 receptor agonists.
Trial registration: ClinicalTrials.gov NCT03021187.
© 2019 by the American Diabetes Association.
Figures

References
-
- Htike ZZ, Zaccardi F, Papamargaritis D, Webb DR, Khunti K, Davies MJ. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: a systematic review and mixed-treatment comparison analysis. Diabetes Obes Metab 2017;19:524–536 - PubMed
-
- Bethel MA, Patel RA, Merrill P, et al. .; EXSCEL Study Group . Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol 2018;6:105–113 - PubMed
-
- American Diabetes Association Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes - 2019. Diabetes Care 2019;42(Suppl. 1):S90–S102 - PubMed
-
- Castellana M, Cignarelli A, Brescia F, Laviola L, Giorgino F. GLP-1 receptor agonist added to insulin versus basal-plus or basal-bolus insulin therapy in type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab Res Rev 2019;35:e3082. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

