Protective Effect of Fat Extract on UVB-Induced Photoaging In Vitro and In Vivo
- PMID: 31531185
- PMCID: PMC6720842
- DOI: 10.1155/2019/6146942
Protective Effect of Fat Extract on UVB-Induced Photoaging In Vitro and In Vivo
Abstract
Background: Nanofat can protect against ultraviolet B- (UVB-) induced damage in nude mice. Fat extract (FE) is a cell-free fraction isolated from nanofat that is enriched with a variety of growth factors.
Objective: To determine whether FE can protect against UVB-induced photoaging in cultured dermal fibroblasts and in nude mice.
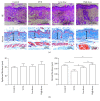
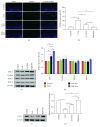
Method: For the in vitro study, human dermal skin fibroblasts were pretreated with FE 24 h prior to UVB irradiation. Generation of reactive oxygen species (ROS) was analyzed immediately following irradiation, while cell cycle analysis was performed 24 h after UVB irradiation. Senescence-associated β-galactosidase (SA-β-gal) expression, cell proliferation, and expression of glutathione peroxidase 1 (GPX-1), catalase, superoxide dismutase-1 (SOD-1), SOD-2, and collagen type 1 (COL-1) were investigated 72 h after UVB irradiation. For the in vivo study, the dorsal skin of nude mice was irradiated with UVB and mice were then treated with FE for 8 weeks. The thickness of the dermis, capillary density, and apoptotic cells in skin tissue sections were investigated after treatment. The expression of GPX-1, catalase, SOD-2, SOD-1, and COL-1 in the tissue was also measured.
Result: FE significantly increased cell proliferation and protected cells against UVB-induced cell death and cell cycle arrest. FE reduced ROS and the number of aged cells induced by UVB irradiation. FE promoted the expression of COL-1 and GPX-1 in cultured dermal fibroblasts. FE treatment of UVB-irradiated skin increased dermal thickness and capillary density, decreased the number of apoptotic cells, and promoted the expression of COL-1 and GPX-1.
Conclusion: FE protects human dermal fibroblasts and the skin of nude mice from UVB-induced photoaging through its antioxidant, antiapoptotic, and proangiogenic activities.
Conflict of interest statement
The authors have declared that there is no conflict of interest.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

