Ketamine effects on default mode network activity and vigilance: A randomized, placebo-controlled crossover simultaneous fMRI/EEG study
- PMID: 31532029
- PMCID: PMC7268043
- DOI: 10.1002/hbm.24791
Ketamine effects on default mode network activity and vigilance: A randomized, placebo-controlled crossover simultaneous fMRI/EEG study
Abstract
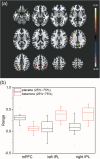
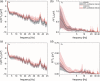
In resting-state functional connectivity experiments, a steady state (of consciousness) is commonly supposed. However, recent research has shown that the resting state is a rather dynamic than a steady state. In particular, changes of vigilance appear to play a prominent role. Accordingly, it is critical to assess the state of vigilance when conducting pharmacodynamic studies with resting-state functional magnetic resonance imaging (fMRI) using drugs that are known to affect vigilance such as (subanesthetic) ketamine. In this study, we sought to clarify whether the previously described ketamine-induced prefrontal decrease of functional connectivity is related to diminished vigilance as assessed by electroencephalography (EEG). We conducted a randomized, double-blind, placebo-controlled crossover study with subanesthetic S-Ketamine in N = 24 healthy, young subjects by simultaneous acquisition of resting-state fMRI and EEG data. We conducted seed-based default mode network functional connectivity and EEG power spectrum analyses. After ketamine administration, decreased functional connectivity was found in medial prefrontal cortex whereas increased connectivities were observed in intraparietal cortices. In EEG, a shift of energy to slow (delta, theta) and fast (gamma) wave frequencies was seen in the ketamine condition. Frontal connectivity is negatively related to EEG gamma and theta activity while a positive relationship is found for parietal connectivity and EEG delta power. Our results suggest a direct relationship between ketamine-induced functional connectivity changes and the concomitant decrease of vigilance in EEG. The observed functional changes after ketamine administration may serve as surrogate end points and provide a neurophysiological framework, for example, for the antidepressant action of ketamine (trial name: 29JN1556, EudraCT Number: 2009-012399-28).
Keywords: EEG-fMRI; antidepressant action; depression; ketamine; vigilance control.
© 2019 The Authors. Human Brain Mapping published by Wiley Periodicals, Inc.
Conflict of interest statement
G.W. is president of Pharmaimage Biomarker Solutions, Inc. (Boston, USA) and CEO of Pharmaimage Biomarker Solutions GmbH (Berlin, Germany):
Figures



Similar articles
-
Pharmacological fMRI: Effects of subanesthetic ketamine on resting-state functional connectivity in the default mode network, salience network, dorsal attention network and executive control network.Neuroimage Clin. 2018 Jun 1;19:745-757. doi: 10.1016/j.nicl.2018.05.037. eCollection 2018. Neuroimage Clin. 2018. PMID: 30003027 Free PMC article. Clinical Trial.
-
Short-term antidepressant administration reduces default mode and task-positive network connectivity in healthy individuals during rest.Neuroimage. 2014 Mar;88:47-53. doi: 10.1016/j.neuroimage.2013.11.022. Epub 2013 Nov 21. Neuroimage. 2014. PMID: 24269575 Clinical Trial.
-
Effects of ketamine and midazolam on resting state connectivity and comparison with ENIGMA connectivity deficit patterns in schizophrenia.Hum Brain Mapp. 2020 Feb 15;41(3):767-778. doi: 10.1002/hbm.24838. Epub 2019 Oct 21. Hum Brain Mapp. 2020. PMID: 31633254 Free PMC article. Clinical Trial.
-
Cortical high-frequency oscillations (≈ 110 Hz) in cats are state-dependent and enhanced by a subanesthetic dose of ketamine.Behav Brain Res. 2025 Jan 5;476:115231. doi: 10.1016/j.bbr.2024.115231. Epub 2024 Aug 30. Behav Brain Res. 2025. PMID: 39218075 Review.
-
[The brain imaging studies of obstructive sleep apnea: evidence from resting-state EEG and fMRI].Sheng Li Xue Bao. 2019 Oct 25;71(5):760-768. Sheng Li Xue Bao. 2019. PMID: 31646330 Review. Chinese.
Cited by
-
Esketamine Combined with Propofol TCI versus Propofol TCI for Deep Sedation during Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: A Prospective, Randomized, and Controlled Trial.Int J Clin Pract. 2023 Dec 11;2023:1155126. doi: 10.1155/2023/1155126. eCollection 2023. Int J Clin Pract. 2023. PMID: 38115950 Free PMC article. Clinical Trial.
-
Anterior default mode network and posterior insular connectivity is predictive of depressive symptom reduction following serial ketamine infusion.Psychol Med. 2022 Sep;52(12):2376-2386. doi: 10.1017/S0033291722001313. Epub 2022 May 17. Psychol Med. 2022. PMID: 35578581 Free PMC article.
-
Whole brain network effects of subcallosal cingulate deep brain stimulation for treatment-resistant depression.Mol Psychiatry. 2024 Jan;29(1):112-120. doi: 10.1038/s41380-023-02306-6. Epub 2023 Nov 2. Mol Psychiatry. 2024. PMID: 37919403 Free PMC article.
-
Ketamine Alters Functional Gamma and Theta Resting-State Connectivity in Healthy Humans: Implications for Schizophrenia Treatment Targeting the Glutamate System.Front Psychiatry. 2021 Jun 10;12:671007. doi: 10.3389/fpsyt.2021.671007. eCollection 2021. Front Psychiatry. 2021. PMID: 34177660 Free PMC article.
-
The Psychedelic Future of Post-Traumatic Stress Disorder Treatment.Curr Neuropharmacol. 2024;22(4):636-735. doi: 10.2174/1570159X22666231027111147. Curr Neuropharmacol. 2024. PMID: 38284341 Free PMC article. Review.
References
-
- Alberti, S. , Chiesa, A. , Andrisano, C. , & Serretti, A. (2015). Insomnia and somnolence associated with second‐generation antidepressants during the treatment of major depression. Journal of Clinical Psychopharmacology, 35, 296–303. - PubMed
-
- Bergouignan, L. , Lemogne, C. , Foucher, A. , Longin, E. , Vistoli, D. , Allilaire, J. F. , & Fossati, P. (2008). Field perspective deficit for positive memories characterizes autobiographical memory in euthymic depressed patients. Behaviour Research and Therapy, 46, 322–333. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

