Association of Serum Ustekinumab Levels With Clinical Response in Psoriasis
- PMID: 31532460
- PMCID: PMC6751771
- DOI: 10.1001/jamadermatol.2019.1783
Association of Serum Ustekinumab Levels With Clinical Response in Psoriasis
Abstract
Importance: High-cost biologic therapies have transformed the management of immune-mediated inflammatory diseases. To optimize outcomes and reduce costs, dose adjustment informed by measurement of circulating drug levels has been shown to be effective in various settings. However, limited evidence exists for this approach with the interleukin 12 and interleukin 23 inhibitor ustekinumab.
Objective: To evaluate clinical utility of therapeutic drug monitoring for ustekinumab in patients with psoriasis.
Design, setting, and participants: A prospective observational cohort of 491 adults with psoriasis was recruited to the multicenter Biomarkers of Systemic Treatment Outcomes in Psoriasis study within the British Association of Dermatologists Biologic and Immunomodulators Register from June 2009 to December 2017; samples from some patients were taken between 2009 and 2011 as part of a pilot study with the same inclusion criteria.
Exposure: Serum ustekinumab level measured at any point during the dosing cycle using an enzyme-linked immunosorbent assay.
Main outcomes and measures: Disease activity measured using the Psoriasis Area and Severity Index (PASI) score. Treatment response outcomes were PASI75 (75% reduction in PASI score from baseline [primary outcome]), PASI90 (90% reduction of PASI score from baseline), and absolute PASI score of 1.5 or less.
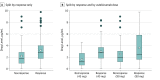
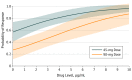
Results: A total of 491 patients (171 women and 320 men; mean [SD] age, 45.7 [12.8] years) had 1 or more serum samples (total, 853 samples obtained 0-56 weeks from start of treatment) and 1 or more PASI scores within the first year of treatment. Antidrug antibodies were detected in only 17 of 490 patients (3.5%). Early measured drug levels (1-12 weeks after starting treatment) were associated with PASI75 response 6 months after starting treatment (odds ratio, 1.38; 95% CI, 1.11-1.71) when adjusted for baseline PASI score, age, and ustekinumab dose. However, this finding was not consistent across the other PASI outcomes (PASI90 and PASI score of ≤1.5).
Conclusions and relevance: This real-world study provides evidence that measurement of early serum ustekinumab levels could be useful to direct the treatment strategy for psoriasis. Adequate drug exposure early in the treatment cycle may be particularly important in determining clinical outcome.
Conflict of interest statement
Figures
Comment in
- doi: 10.1001/jamadermatol.2019.2587
References
-
- Parisi R, Symmons DP, Griffiths CE, Ashcroft DM; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team . Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377-385. doi:10.1038/jid.2012.339 - DOI - PubMed
-
- Warren RB, Smith CH, Yiu ZZN, et al. . Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol. 2015;135(11):2632-2640. doi:10.1038/jid.2015.208 - DOI - PubMed
-
- electronic Medicines Compendium Stelara 45 mg solution for injection (vials). https://www.medicines.org.uk/emc/product/4413/smpc. Accessed January 1, 2019.

