Prevalence and management of drug-drug interactions with antiretroviral treatment in 2069 people living with HIV in rural Tanzania: a prospective cohort study
- PMID: 31532898
- PMCID: PMC6916175
- DOI: 10.1111/hiv.12801
Prevalence and management of drug-drug interactions with antiretroviral treatment in 2069 people living with HIV in rural Tanzania: a prospective cohort study
Abstract
Objectives: Widespread access to antiretroviral therapy (ART) has substantially increased life expectancy in sub-Saharan African countries. As a result, the rates of comorbidities and use of co-medications among people living with HIV are increasing, necessitating a sound understanding of drug-drug interactions (DDIs). We aimed to assess the prevalence and management of DDIs with ART in a rural Tanzanian setting.
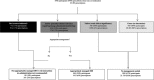
Methods: We included consenting HIV-positive adults initiating ART in the Kilombero and Ulanga Antiretroviral Cohort (KIULARCO) between January 2013 and December 2016. DDIs were classified using www.hiv-druginteractions.org as red (contra-indicated), amber (potential clinical relevance requiring dosage adjustment/monitoring), yellow (weak clinical significance unlikely to require further management) or green (no interaction). We assessed management of amber DDIs by evaluating monitoring of laboratory or clinical parameters, or changes in drug dosages.
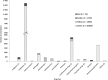
Results: Of 2069 participants, 1945 (94%) were prescribed at least one co-medication during a median follow-up of 1.8 years. Of these, 645 (33%) had at least one potentially clinically relevant DDI, with the highest grade being red in nine (< 1%) and amber in 636 (33%) participants. Of the 23 283 prescriptions, 19 (< 1%) and 1745 (7%) were classified as red and amber DDIs, respectively. Overall, 351 (2%) prescriptions were red DDIs or not appropriately managed amber DDIs.
Conclusions: Co-medication use was common in this rural sub-Saharan cohort. A third of participants had DDIs requiring further management. Of the 9% of participants with not appropriately managed DDIs, most were with cardiovascular and analgesic drugs. This highlights the importance of physicians' awareness of DDIs for their recognition and management.
Keywords: HIV infection; drug-drug interaction; drug-drug interaction management; sub-Saharan Africa.
© 2019 The Authors. HIV Medicine published by John Wiley & Sons Ltd on behalf of British HIV Association.
Figures
References
-
- World Health Organisation (WHO) . WHO | Global AIDS Update [Internet], 2016. Available at http://www.who.int/hiv/pub/arv/global-aids-update-2016-pub/en/ (accessed 01 May 2019).
-
- UNAIDS . Global Report 2013 [Internet], 2013. Available at http://www.unaids.org/en/resources/campaigns/globalreport2013/globalreport (accessed 01 May 2019).
-
- World Health Organization . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. [Internet], 2016. Available at http://www.ncbi.nlm.nih.gov/books/NBK374294/ (accessed 1 April 2017). - PubMed
-
- Seden K, Back D, Khoo S. Antiretroviral drug interactions: often unrecognized, frequently unavoidable, sometimes unmanageable. J Antimicrob Chemother 2009; 64: 5–8. - PubMed
-
- Seden K, Khoo SH, Back D et al Global patient safety and antiretroviral drug‐drug interactions in the resource‐limited setting. J Antimicrob Chemother 2013; 68: 1–3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

