TCR and Inflammatory Signals Tune Human MAIT Cells to Exert Specific Tissue Repair and Effector Functions
- PMID: 31533032
- PMCID: PMC6899450
- DOI: 10.1016/j.celrep.2019.08.050
TCR and Inflammatory Signals Tune Human MAIT Cells to Exert Specific Tissue Repair and Effector Functions
Abstract
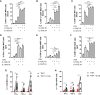
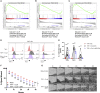
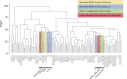
MAIT cells are an unconventional T cell population that can be activated through both TCR-dependent and TCR-independent mechanisms. Here, we examined the impact of combinations of TCR-dependent and TCR-independent signals in human CD8+ MAIT cells. TCR-independent activation of these MAIT cells from blood and gut was maximized by extending the panel of cytokines to include TNF-superfamily member TL1A. RNA-seq experiments revealed that TCR-dependent and TCR-independent signals drive MAIT cells to exert overlapping and specific effector functions, affecting both host defense and tissue homeostasis. Although TCR triggering alone is insufficient to drive sustained activation, TCR-triggered MAIT cells showed specific enrichment of tissue-repair functions at the gene and protein levels and in in vitro assays. Altogether, these data indicate the blend of TCR-dependent and TCR-independent signaling to CD8+ MAIT cells may play a role in controlling the balance between healthy and pathological processes of tissue inflammation and repair.
Keywords: MAIT cells; TCR signaling; cytokines; effector functions; tissue repair.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures



Comment in
-
A TCR-Dependent Tissue Repair Potential of MAIT Cells.Trends Immunol. 2019 Nov;40(11):975-977. doi: 10.1016/j.it.2019.09.001. Epub 2019 Oct 14. Trends Immunol. 2019. PMID: 31623980
References
-
- Bamias G., Martin C., 3rd, Marini M., Hoang S., Mishina M., Ross W.G., Sachedina M.A., Friel C.M., Mize J., Bickston S.J. Expression, localization, and functional activity of TL1A, a novel Th1-polarizing cytokine in inflammatory bowel disease. J. Immunol. 2003;171:4868–4874. - PubMed
-
- Billerbeck E., Kang Y.H., Walker L., Lockstone H., Grafmueller S., Fleming V., Flint J., Willberg C.B., Bengsch B., Seigel B. Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc. Natl. Acad. Sci. USA. 2010;107:3006–3011. - PMC - PubMed
-
- Boniface K., Diveu C., Morel F., Pedretti N., Froger J., Ravon E., Garcia M., Venereau E., Preisser L., Guignouard E. Oncostatin M secreted by skin infiltrating T lymphocytes is a potent keratinocyte activator involved in skin inflammation. J. Immunol. 2007;178:4615–4622. - PubMed
-
- Chen Z., Wang H., D’Souza C., Sun S., Kostenko L., Eckle S.B.G., Meehan B.S., Jackson D.C., Strugnell R.A., Cao H. Mucosal-associated invariant T-cell activation and accumulation after in vivo infection depends on microbial riboflavin synthesis and co-stimulatory signals. Mucosal Immunol. 2017;10:58–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

