Spatial confidence sets for raw effect size images
- PMID: 31533067
- PMCID: PMC6854455
- DOI: 10.1016/j.neuroimage.2019.116187
Spatial confidence sets for raw effect size images
Abstract
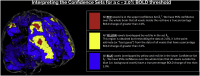
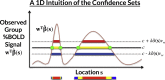
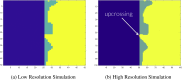
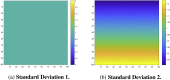
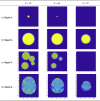
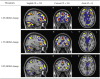
The mass-univariate approach for functional magnetic resonance imaging (fMRI) analysis remains a widely used statistical tool within neuroimaging. However, this method suffers from at least two fundamental limitations: First, with sufficient sample sizes there is high enough statistical power to reject the null hypothesis everywhere, making it difficult if not impossible to localize effects of interest. Second, with any sample size, when cluster-size inference is used a significant p-value only indicates that a cluster is larger than chance. Therefore, no notion of confidence is available to express the size or location of a cluster that could be expected with repeated sampling from the population. In this work, we address these issues by extending on a method proposed by Sommerfeld et al. (2018) (SSS) to develop spatial Confidence Sets (CSs) on clusters found in thresholded raw effect size maps. While hypothesis testing indicates where the null, i.e. a raw effect size of zero, can be rejected, the CSs give statements on the locations where raw effect sizes exceed, and fall short of, a non-zero threshold, providing both an upper and lower CS. While the method can be applied to any mass-univariate general linear model, we motivate the method in the context of blood-oxygen-level-dependent (BOLD) fMRI contrast maps for inference on percentage BOLD change raw effects. We propose several theoretical and practical implementation advancements to the original method formulated in SSS, delivering a procedure with superior performance in sample sizes as low as N=60. We validate the method with 3D Monte Carlo simulations that resemble fMRI data. Finally, we compute CSs for the Human Connectome Project working memory task contrast images, illustrating the brain regions that show a reliable %BOLD change for a given %BOLD threshold.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Figures











References
-
- Hariri Ahmad R., Tessitore Alessandro, Mattay Venkata S., Fera Francesco, Weinberger Daniel R. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. September 2002;17(1):317–323. - PubMed
-
- Alfaro-Almagro Fidel, Jenkinson Mark, Bangerter Neal K., Andersson Jesper L.R., Griffanti Ludovica, Douaud Gwenaëlle, Sotiropoulos Stamatios N., Jbabdi Saad, Hernandez-Fernandez Moises, Vallee Emmanuel, Vidaurre Diego, Webster Matthew, McCarthy Paul, Rorden Christopher, Daducci Alessandro, Alexander Daniel C., Zhang Hui, Dragonu Iulius, Matthews Paul M., Miller Karla L., Smith Stephen M. Image processing and quality control for the first 10,000 brain imaging datasets from UK biobank. Neuroimage. February 2018;166:400–424. - PMC - PubMed
-
- Barch Deanna M., Burgess Gregory C., Harms Michael P., Petersen Steven E., Schlaggar Bradley L., Corbetta Maurizio, Glasser Matthew F., Curtiss Sandra, Dixit Sachin, Feldt Cindy, Nolan Dan, Bryant Edward, Tucker Hartley, Owen Footer, Bjork James M., Poldrack Russ, Smith Steve, Johansen-Berg Heidi, Snyder Abraham Z., Van Essen David C., WU-Minn HCP Consortium Function in the human connectome: task-fMRI and individual differences in behavior. Neuroimage. October 2013;80:169–189. - PMC - PubMed
-
- Chernozhukov Victor, Chetverikov Denis, Kato Kengo. 2013. Gaussian Approximations and Multiplier Bootstrap for Maxima of Sums of High-Dimensional Random Vectors.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

