Electrical and Electrochemical Behavior of Carbon Paste Electrodes Modified with Ionic Liquids Based in N-Octylpyridinium Bis(Trifluoromethylsulfonyl)Imide. A Theoretical and Experimental Study
- PMID: 31533331
- PMCID: PMC6767309
- DOI: 10.3390/molecules24183382
Electrical and Electrochemical Behavior of Carbon Paste Electrodes Modified with Ionic Liquids Based in N-Octylpyridinium Bis(Trifluoromethylsulfonyl)Imide. A Theoretical and Experimental Study
Abstract
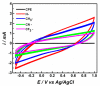
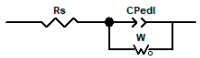
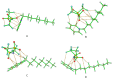
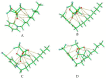
In this work, we studied carbon paste electrodes (CPEs) with two kinds of binders: mineral oil or ionic liquids (IL) derived from N-substituted octyl pyridinium bis(trifluoromethylsulfonyl)imide with the substituents H-, CH3-, CN- and CF3-. The work aims to study this series of IL and determine a possible effect of the substituent of the cation in the behavior of the IL as a binder of graphite for obtaining IL-CPEs. The electrochemical response and the electrical behavior were measured by cyclic voltammetry and electrochemical impedance spectroscopy, respectively. Surprisingly, the substituent does not affect the cyclic voltammetry response because in all the cases, high resistance and high capacitive currents were obtained. The best response in terms of conductivity is obtained by CPE. In the case of impedance measurements, the substituent does not cause differences, and in all the cases, the IL-CPEs show nearly the same responses. CPE shows lower capacitance and higher resistance for diffusion compared to the IL-CPEs due to his lower porosity. The high resistance showed by the IL-CPEs by cyclic voltammetry can be attributed to poorly intermolecular forces among graphite, water, electrolyte, and ILs as demonstrated by theoretical calculations.
Keywords: N-octyl pyridinium bis(trifluoromethylsulfonyl)imide; intramolecular forces of ionic liquid; ionic-liquid carbon paste electrodes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




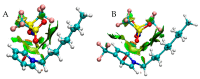
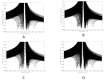
References
-
- Plechkova N.V., Seddon K.R. Ionic Liquids: “Designer” Solvents for Green Chemistry. In: Tundo P., Perosa A., Zecchini F., editors. Methods and Reagents for Green Chemistry. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2007. pp. 103–130.
-
- Huang B., Wang P., Hao W., Cai M.-Z. The first example of hydrozirconation of alkynes in room temperature ionic liquids. J. Organomet. Chem. 2011;696:2685–2688. doi: 10.1016/j.jorganchem.2011.04.002. - DOI
-
- Garcia Bernal E., De Los Rios A. P., Hernandez F. J., Larrosa-Guerrero A., Ginestá A., Sánchez S., Lozano L. J. Aplicaciones de los líquidos iónicos en la industria química. IV Jornadas Introd. Invetigacion UPCT. 2011;4:66–68.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

