Identification and characterization of NF1 and non-NF1 congenital pseudarthrosis of the tibia based on germline NF1 variants: genetic and clinical analysis of 75 patients
- PMID: 31533797
- PMCID: PMC6751843
- DOI: 10.1186/s13023-019-1196-0
Identification and characterization of NF1 and non-NF1 congenital pseudarthrosis of the tibia based on germline NF1 variants: genetic and clinical analysis of 75 patients
Abstract
Background: Congenital pseudarthrosis of the tibia (CPT) is a rare disease. Some patients present neurofibromatosis type 1 (NF1), while some others do not manifest NF1 (non-NF1). The etiology of CPT, particularly non-NF1 CPT, is not well understood. Here we screened germline variants of 75 CPT cases, including 55 NF1 and 20 non-NF1. Clinical data were classified and analyzed based on NF1 gene variations to investigate the genotype-phenotype relations of the two types of patients.
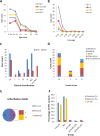
Results: Using whole-exome sequencing and Multiplex Ligation-Dependent Probe Amplification, 44 out of 55 NF1 CPT patients (80.0%) were identified as carrying pathogenic variants of the NF1 gene. Twenty-five variants were novel; 53.5% of variants were de novo, and a higher proportion of their carriers presented bone fractures compared to inherited variant carriers. No NF1 pathogenic variants were found in all 20 non-NF1 patients. Clinical features comparing NF1 CPT to non-NF1 CPT did not show significant differences in bowing or fracture onset, lateralization, tissue pathogenical results, abnormality of the proximal tibial epiphysis, and follow-up tibial union after surgery. A considerably higher proportion of non-NF1 patients have cystic lesion (Crawford type III) and used braces after surgery.
Conclusions: We analyzed a large cohort of non-NF1 and NF1 CPT patients and provided a new perspective for genotype-phenotype features related to germline NF1 variants. Non-NF1 CPT in general had similar clinical features of the tibia as NF1 CPT. Germline NF1 pathogenic variants could differentiate NF1 from non-NF1 CPT but could not explain the CPT heterogeneity of NF1 patients. Our results suggested that non-NF1 CPT was probably not caused by germline NF1 pathogenic variants. In addition to NF1, other genetic variants could also contribute to CPT pathogenesis. Our findings would facilitate the interpretation of NF1 pathogenic variants in CPT genetic counseling.
Keywords: Genomic variation; Genotype; Neurofibromatosis 1; Phenotype; Whole exome sequencing.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Case series of congenital pseudarthrosis of the tibia unfulfilling neurofibromatosis type 1 diagnosis: 21% with somatic NF1 haploinsufficiency in the periosteum.Hum Genet. 2022 Aug;141(8):1371-1383. doi: 10.1007/s00439-021-02429-2. Epub 2022 Jan 13. Hum Genet. 2022. PMID: 35024939
-
Unraveling the molecular landscape of congenital pseudoarthrosis of the tibia: insights from a comprehensive analysis of 159 probands.Orphanet J Rare Dis. 2025 Jun 3;20(1):269. doi: 10.1186/s13023-025-03759-4. Orphanet J Rare Dis. 2025. PMID: 40462134 Free PMC article.
-
[The clinical characteristics of 497 children with congenital pseudarthrosis of the tibia].Zhonghua Wai Ke Za Zhi. 2024 Sep 1;62(9):864-869. doi: 10.3760/cma.j.cn112139-20240328-00150. Zhonghua Wai Ke Za Zhi. 2024. PMID: 39090065 Chinese.
-
Prevalence of neurofibromatosis type 1 in congenital pseudarthrosis of the tibia.Eur J Pediatr. 2016 Sep;175(9):1193-1198. doi: 10.1007/s00431-016-2757-z. Epub 2016 Aug 12. Eur J Pediatr. 2016. PMID: 27519821 Review.
-
A benign form of congenital anterolateral bowing of the tibia associated with ipsilateral polydactyly of the hallux: case report and literature review.Am J Med Genet A. 2012 Jul;158A(7):1742-9. doi: 10.1002/ajmg.a.35417. Epub 2012 Jun 7. Am J Med Genet A. 2012. PMID: 22678991 Review.
Cited by
-
Neurofibromatosis type 1: Clinical characteristics and mutation spectrum in a North Indian cohort.Heliyon. 2023 Dec 21;10(1):e23685. doi: 10.1016/j.heliyon.2023.e23685. eCollection 2024 Jan 15. Heliyon. 2023. PMID: 38226287 Free PMC article.
-
Anterolateral Tibial Bowing and Congenital Pseudoarthrosis of the Tibia: Current Concept Review and Future Directions.Curr Rev Musculoskelet Med. 2022 Dec;15(6):438-446. doi: 10.1007/s12178-022-09779-y. Epub 2022 Jul 16. Curr Rev Musculoskelet Med. 2022. PMID: 35841513 Free PMC article. Review.
-
Are Children Suffering From Congenital Pseudarthrosis of the Tibia Associated With Decreased Bone Strength?Front Pediatr. 2022 May 9;10:859580. doi: 10.3389/fped.2022.859580. eCollection 2022. Front Pediatr. 2022. PMID: 35615635 Free PMC article.
-
Differential expression and effect analysis of lncRNA-mRNA in congenital pseudarthrosis of the tibia.Front Genet. 2023 Feb 6;14:1094298. doi: 10.3389/fgene.2023.1094298. eCollection 2023. Front Genet. 2023. PMID: 36814904 Free PMC article.
-
Hybrid Minigene Assay: An Efficient Tool to Characterize mRNA Splicing Profiles of NF1 Variants.Cancers (Basel). 2021 Feb 27;13(5):999. doi: 10.3390/cancers13050999. Cancers (Basel). 2021. PMID: 33673681 Free PMC article.
References
-
- Crawford AH. Neurofibromatosis in children. Acta Orthop Scand Suppl. 1986;218:1–60. - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

