Elevated TRIP13 drives the AKT/mTOR pathway to induce the progression of hepatocellular carcinoma via interacting with ACTN4
- PMID: 31533816
- PMCID: PMC6749659
- DOI: 10.1186/s13046-019-1401-y
Elevated TRIP13 drives the AKT/mTOR pathway to induce the progression of hepatocellular carcinoma via interacting with ACTN4
Erratum in
-
Correction to: Elevated TRIP13 drives the AKT/mTOR pathway to induce the progression of hepatocellular carcinoma via interacting with ACTN4.J Exp Clin Cancer Res. 2019 Oct 31;38(1):443. doi: 10.1186/s13046-019-1454-y. J Exp Clin Cancer Res. 2019. PMID: 31666112 Free PMC article.
Abstract
Background: ATPase associated with a variety of cellular activities (AAA ATPase) family members are closely linked to tumor formation and progression. However, their roles in hepatocellular carcinoma (HCC) largely remain unclear.
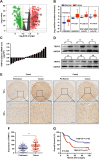
Methods: Bioinformatic analyses of public databases were used to excavate the potential AAA ATPases that may contribute to HCC, and thyroid hormone receptor interactor 13 (TRIP13) was selected to following researches because of its most prominently differential expression. Western blot, qRT-PCR and immunohistochemistry were used to detect the expression of TRIP13 in HCC tissues, and then the relationship between TRIP13 expression and clinicopathological parameters were evaluated. Finally, its functions and potential mechanisms were investigated through a series gain- and loss-of-function strategies both in vitro and in vivo.
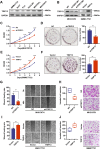
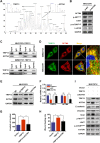
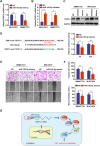
Results: TRIP13 was significantly overexpressed in HCC tissues and high level of TRIP13 was closely correlated with a worse clinical outcome. Functionally, elevated TRIP13 facilitated cell proliferation, migration, invasion, and promoted cellular epithelial-mesenchymal transition (EMT) in vitro, while promote tumor growth and lung metastasis in vivo. Mechanistically, TRIP13 interacted with ACTN4 and positively regulated its expression, thus activating the AKT/mTOR pathway to drive tumor progression. Moreover, miR-192-5p served as an upstream regulator of TRIP13 by directly binding to TRIP13 mRNA 3' UTR, which may partially explain the high expression of TRIP13 in HCC.
Conclusion: Our findings identified TRIP13 as a promising candidate oncogene in HCC, and TRIP13 induced cell migration, invasion and metastasis of HCC through the AKT/mTOR signaling via interacting with ACTN4.
Keywords: ACTN4; EMT; HCC; TRIP13; miR-192-5p.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Neuwald AF, Aravind L, Spouge JL, Koonin EV. AAA+: a class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 1999;9(1):27–43. - PubMed
-
- Mikesch Jan-Henrik, Schwammbach Daniela, Hartmann Wolfgang, Schmidt Lars H., Schliemann Christoph, Angenendt Linus, Wiewrodt Rainer, Marra Alessandro, Thoennissen Nils H., Wardelmann Eva, Köhler Gabriele, Lenz Georg, Müller-Tidow Carsten, Berdel Wolfgang E., Arteaga Maria-Francisca. Reptin drives tumour progression and resistance to chemotherapy in nonsmall cell lung cancer. European Respiratory Journal. 2018;52(1):1701637. doi: 10.1183/13993003.01637-2017. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

