Targeting interferon signaling and CTLA-4 enhance the therapeutic efficacy of anti-PD-1 immunotherapy in preclinical model of HPV+ oral cancer
- PMID: 31533840
- PMCID: PMC6749627
- DOI: 10.1186/s40425-019-0728-4
Targeting interferon signaling and CTLA-4 enhance the therapeutic efficacy of anti-PD-1 immunotherapy in preclinical model of HPV+ oral cancer
Abstract
Background: The US is experiencing an epidemic of HPV+ oropharyngeal cancers (OPC), the rates and burden of which now exceed that for cervical cancer. Immunotherapy targeting programmed death 1 (PD-1) on tumor-infiltrating lymphocytes and/or its ligand PD-L1 on tumor cells, which was effective in several cancers has however, showed efficacy in only less than 15% of patients.
Methods: We used a preclinical HPV+ oral tumor model, mEER, consisting of mouse tonsil derived epithelial cells expressing HPV-16 E6 and E7 genes, along with the H-ras oncogene to test strategies for enhancing the efficacy of anti-PD-1 therapy.
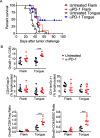
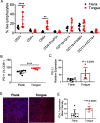
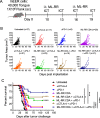
Results: Monotherapy with PD-1 blocking antibody was ineffective against flank-implanted tumors, but induced regression in 54% of mice bearing orthotopic tongue tumors that correlated with higher CD8 T cell responses. Since the CD8+ T cells derived from tongue tumors also showed high levels of the immune checkpoint inhibitory receptor CTLA-4, we tested combination immunotherapy targeting both CTLA-4 and PD-1 together and observed 93.3% survival of mice bearing tumors in the tongue for the duration of our 100-day study. Protective immunity correlated with a significant decrease in immunosuppressive lymphoid and myeloid populations within the tumor microenvironment. Consistent with the reported capacity of interferon-driven PD-L1/PD-1 pathway induction to serve as a biomarker of response to PD-1 blockade, we observed elevated interferon signaling and significantly higher levels of PD-1/PD-L1 in tongue-implanted mEER tumors compared to those growing on the flank correlating with their preferential responsiveness to PD-1 blockade. More importantly, in a pseudometastasic mouse model bearing both flank and tongue tumors to represent metastatic disease, delivery of Stimulator of Interferon Induced Genes (STING) agonist into the flank tumors combined with systemic treatment with α-PD-1 and α-CTLA-4 antibodies resulted in sustained tumor regression in 71% of mice. In this case, productive abscopal anti-tumor immunity was associated with robust increases in the ratios of cytotoxic CD8+ T cells (CTL) versus regulatory T cells (Treg) and versus functional myeloid-derived suppressor cells (MDSC).
Conclusions: These results support combining α-PD-1 therapy with induction of IFN-α/β signaling via provision of STING agonist and/or through CTLA-4 blockade as potential treatment option for HNSCC patients, especially, those not responding to α-PD-1 monotherapy.
Keywords: Abscopal; CD8+ T cells; Checkpoint blockade; HNSCC; HPV; Immunotherapy; MDSC; STING.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
