Hemoadsorption with CytoSorb shows a decreased observed versus expected 28-day all-cause mortality in ICU patients with septic shock: a propensity-score-weighted retrospective study
- PMID: 31533846
- PMCID: PMC6749645
- DOI: 10.1186/s13054-019-2588-1
Hemoadsorption with CytoSorb shows a decreased observed versus expected 28-day all-cause mortality in ICU patients with septic shock: a propensity-score-weighted retrospective study
Abstract
Background and aims: Innovative treatment modalities have not yet shown a clinical benefit in patients with septic shock. To reduce severe cytokinaemia, CytoSorb as an add-on to continuous renal replacement therapy (CRRT) showed promising results in case reports. However, there are no clinical trials investigating outcomes.
Methods: In this investigator-initiated retrospective study, patients with septic shock were treated with CRRT + CytoSorb (n = 67) or CRRT alone (n = 49). The primary outcome was the 28-day all-cause mortality rate. Patients were weighted by stabilized inverse probability of treatment weights (sIPTW) to overcome differences in baseline characteristics.
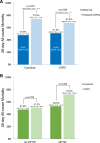
Results: At the start of therapy, CytoSorb-treated patients had higher lactate levels (p < 0.001), lower mean arterial pressure (p = 0.007) and higher levels of noradrenaline (p < 0.001) compared to the CRRT group. For CytoSorb, the mean predicted mortality rate based on a SOFA of 13.8 (n = 67) was 75% (95%CI 71-79%), while the actual 28-day mortality rate was 48% (mean difference - 27%, 95%CI - 38 to - 15%, p < 0.001). For CRRT, based on a SOFA of 12.8 (n = 49), the mean predicted versus observed mortality was 68% versus 51% (mean difference - 16.9% [95%CI - 32.6 to - 1.2%, p = 0.035]). By sIPTW analysis, patients treated with CytoSorb had a significantly lower 28-day mortality rate compared to CRRT alone (53% vs. 72%, respectively, p = 0.038). Independent predictors of 28-day mortality in the CytoSorb group were the presence of pneumosepsis (adjusted odds ratio [aOR] 5.47, p = 0.029), higher levels of lactate at the start of CytoSorb (aOR 1.15, p = 0.031) and older age (aOR per 10 years 1.67, p = 0.034).
Conclusions: CytoSorb was associated with a decreased observed versus expected 28-day all-cause mortality. By IPTW analysis, intervention with CytoSorb may be associated with a decreased all-cause mortality at 28 days compared to CRRT alone.
Keywords: Cytosorb; Cytosorbent; Hemofiltration; Mortality; Outcome; Sepsis; Septic shock; Treatment.
Conflict of interest statement
WPB, MK and SD declare that they have no competing interests. CI has received a grant from CytoSorb to commence a randomized controlled trial on the effect of the adsorber on the microcirculation of critically ill patients at the department of Intensive Care of the Erasmus Medical Center Rotterdam.
Figures
Comment in
-
Hemoadsorption of cytokines by CytoSorb filter: a simulation study without human factor-pilot is the difference.Crit Care. 2020 Jan 13;24(1):13. doi: 10.1186/s13054-019-2716-y. Crit Care. 2020. PMID: 31931850 Free PMC article. No abstract available.
References
-
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. - DOI - PMC - PubMed
-
- Vincent JL, Marshall JC, Namendys-Silva SA, Francois B, Martin-Loeches I, Lipman J, Reinhart K, Antonelli M, Pickkers P, Njimi H, et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2(5):380–386. doi: 10.1016/S2213-2600(14)70061-X. - DOI - PubMed
-
- Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193(3):259–272. doi: 10.1164/rccm.201504-0781OC. - DOI - PubMed
-
- Levy MM, Artigas A, Phillips GS, Rhodes A, Beale R, Osborn T, Vincent JL, Townsend S, Lemeshow S, Dellinger RP. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis. 2012;12(12):919–924. doi: 10.1016/S1473-3099(12)70239-6. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical